Chapter 79 Drug Interactions and Administration
Pharmacology plays a critical role in the treatment of HIV infection affecting issues as diverse as adherence, efficacy, and tolerability. The era of HAART has been characterized by drug regimens that are potent but sometimes complicated, burdensome, and possessing a number of long-term toxicities. Most treated patients currently take at least three antiretrovirals, but may also take drugs for prophylaxis and treatment of opportunistic infections, and a variety of medications for supportive care of pain, depression, and other concomitant illnesses. Some patients are also receiving investigational agents and/or alternative medications from health food stores. While great strides have been made to improve formulations and reduce pill burden, the late-stage patient may still require in excess of 10 or more medications and administration of 30–40 pills each day.
FOOD EFFECTS
Food alters the absorption or bioavailability of several drugs commonly used in HIV-infected patients. Drug–food interactions can influence the scheduling of medications during the day, and can profoundly affect the quality of life of patients. Dosing of antiretrovirals can be very confusing for patients, who often have to schedule their meals around drug administration. For example, didanosine (ddI) tablets and sachets are formulated with a buffer that requires administration on an empty stomach because a low gastric pH leads to degradation of the drug.1 Although not buffered, ddI enteric-coated capsules also must be given on an empty stomach since administration with food decreases the Cmax and area under the concentration-time curve (AUC) by 46% and 19%, respectively.2 Bioavailability of the other nucleosides is not significantly affected by food, although peak absorption may be delayed. For example, a high-fat3 or high-protein4 meal slows the rate of absorption of zidovudine, and may reduce the mean maximum concentration, but does not significantly alter the overall extent of absorption.
Several protease inhibitor (PI) regimens are particularly problematic in terms of food effects. When used alone, indinavir (IDV) must be given on an empty stomach or with a light meal containing less than 2 g of fat.5 When combined with ritonavir (RTV), IDV can be administered without regard to food.6 Conversely, nelfinavir requires administration with a meal that contains a minimum of 500 kcal with 20% fat for adequate absorption.7 Tipranavir (TPV) and atazanavir (ATV) absorption are increased by 31% and 70%, respectively, and therefore should be administered with food.8,9 On the other hand, lopinavir (LPV) and fosamprenavir (FPV) tablets are not significantly affected by food, and therefore may be taken without regard to meals.10,11 While RTV capsule absorption is only increased by 15% in the presence of food, administration with a meal is recommended to decrease its gastrointestinal side effects.12 A variety of HIV-related medications have specific food restrictions stemming from the possibility of increased or decreased absorption from the gastrointestinal tract; these restrictions are summarized in Table 79-1. A review of food restrictions should be an important part of patient counseling for a new or modified drug regimen.
Table 79-1 Food–Drug Interactions with HIV-Related Medications
| Antiretrovirals | Food Effect | Recommendation |
|---|---|---|
| Atazanavir | AUC increased by 70% with light meal | Administer with food |
| Amprenavir | AUC decreased by 21% with high-fat meal | Take with or without food but high-fat meal should be avoided |
| Didanosine EC | AUC decreased by 18–27% with food | Administer 2 h before or 2 h after meals |
| Efavirenz | AUC increased by 22% and 50% with low-and high-fat meals | May be taken with or without meals but administer on an empty stomach during the first 2 weeks to minimize the risk of CNS side effects |
| Fosamprenavir | Not affected by food | May be taken with or without meals |
| Indinavir | High-fat caloric meal results in 77% decrease in AUC | May be taken with food when co-administered with ritonavir. Must be administered on empty stomach or with low-fat, light meal if not boosted with ritonavir |
| Lopinavir/r | Lopinavir tablets are not affected by food | Administer with or without food |
| Nelfinavir | AUC two- to fivefold higher when given with food | Administer with a minimum of 500 kcal with 20% fat meal |
| Saquinavir | Marked increase in AUC following high-fat meal | Administer within 2 h of a full meal. Food requirement is less of a factor with ritonavir co-administration |
| Tipranavir/r | AUC increased by 31% with high fat meal | Administer with food |
| Drugs for OIs | ||
| Atovaquone | High-fat meal increases bioavailability up to threefold | Administer with food |
| Valganciclovir | AUC increased by 30% with food | Administer with food |
| Clarithromycin XL | AUC increased by 30% with food | Administer clarithromycin XL with food. |
| Immediate-release clarithromycin can be taken with or without food | ||
| Isoniazid | Reduced absorption with food | Administer on an empty stomach |
| Itraconazole (caps) | Significant increase in bioavailability when taken with a full meal | Administer with food |
| Itraconazole (solution) | Maximal absorption when taken under fasting conditions | Administer without food |
| Ribavirin | Increased absorption with fatty food | Administer with food |
| Rifapentine | AUC increased by 43% | Administer with food |
| Voriconazole | AUC decreased by 24% with food | Administer 1 h before or 1 h after meals |
AUC, area under the concentration–time curve.
SPACING OF MEDICATIONS
The specific administration times for some antiretrovirals, and how they are taken in relation to other medications, may affect their absorption and activity. For example, the antacids in ddI buffered sachets decrease the absorption of IDV and ATV by over 80% if they are administered concomitantly.8,13 Thus, it is recommended to take ddI buffered powder 2 h before the administration of ATV or IDV.8,14 ddI enteric-coated (EC) tablets are preferred, since they do not alter IDV’s or ATV’s absorption and can be given concomitantly.15,16
Concentrations of some PIs in dual combination may differ depending on whether they are given concomitantly or in a staggered fashion. ACTG 378 was a study in healthy volunteers that evaluated single doses of dual combinations of PIs (saquinavir (SQV), RTV, or nelfinavir) given either simultaneously or when separated by 4 h. The AUC of SQV was markedly lower when given 4 h before RTV or nelfinavir; however, if RTV was given up to 48 h before SQV, the levels of SQV were still significantly increased.17 Dose separation of dual PI combinations is not recommended since this may increase the complexity of the antiretroviral regimen in addition to adversely affecting their pharmacokinetic profiles. A number of dual PI combinations can be dosed once-daily (i.e., ATV/ritonavir (r), FPV/r, LPV/r) in antiretroviral-naive patients.8,11,18 Such regimens would be particularly useful for selected populations such as prison inmates, methadone clinics, and those patients requiring directly observed therapy. Although these once-daily regimens would be more convenient, they might also require near-perfect adherence, since concentrations at the end of the 24-h dosing interval may be near the IC50 value. Therefore, a missed dose would result in suboptimal concentrations for a prolonged period, leading to increased risk of recurrent viral replication and drug resistance. Most PIs possess large interpatient and intrapatient pharmacokinetic variability, so that even with good adherence there may be a small proportion of patients with concentrations below the desired target at the end of a dosing interval. Although PK variability of PIs is minimized with the co-administration of RTV, it can still be quite large.5
DRUG INTERACTIONS
One of the greatest challenges for the HIV clinician is the recognition and management of drug interactions.19 The HIV-infected patient often receives numerous medications and has a great potential for adverse drug interactions. Certain PI combinations may also be used to take advantage of beneficial pharmacokinetic interactions.
A number of medications used in HIV-infected patients can produce adverse drug interactions (Table 79-2).19 In addition to over 300 drug interactions listed in the product labeling of antiretrovirals, there are potential interactions with other therapeutic classes not yet studied.7–11,20–31 Although many of these interactions may be minor in nature, some are potentially serious, leading to severe toxicity or treatment failure. Fortunately, many interactions can be recognized, prevented, and corrected. Minor alterations in scheduling or selection of an appropriate alternative are usually all that is required to avoid a potential interaction. Other drug interactions may be beneficial by increasing plasma concentrations of co-administered PIs.
Pharmacokinetic Interactions
Altered Drug Absorption
Impairment of drug absorption can lead to a marked reduction in the bioavailability of certain agents. One of the most common interactions affecting drug absorption is chelation, the binding of drugs to metal ions or other substances in the gastrointestinal tract. The concomitant administration of a fluoroquinolone antibiotic with a di- or trivalent cation such as calcium, magnesium, aluminum, or iron results in a greater than 90% decrease in the AUC of the fluoroquinolone, possibly leading to therapeutic failure.32–34 ddI formulations with a calcium and magnesium buffer (tablets) or citrate-phosphate buffer (sachet) can also alter fluoroquinolone disposition. Concomitant administration of ddI with ciprofloxacin has been shown to decrease the ciprofloxacin AUC from 15.5 to 0.26 mg h−1 mL−1.35 A similar interaction would be expected with other products such as antacids, sucralfate, or iron preparations. Administration of these agents should be separated from the fluoroquinolone by at least 2 h, and the fluoroquinolone should be administered first, followed by the cation, to ensure adequate absorption. The newer EC formulation of ddI does not alter ciprofloxacin pharmacokinetics and can be given concomitantly.2
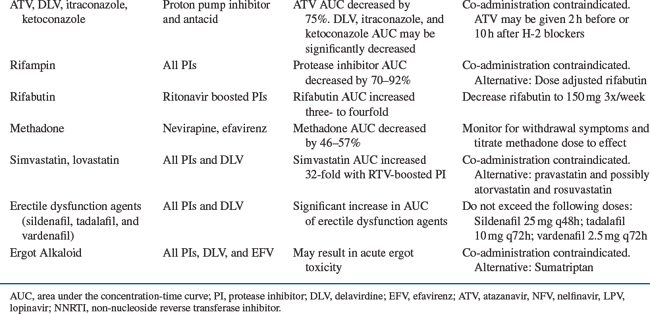
An increase in gastric pH significantly affects the absorption of azole antifungals (i.e., itraconazole) and ATV. An acidic environment is required for dissolution and absorption of ketoconazole, itraconazole, and ATV; thus concomitant administration with histamine-2 (H-2) antagonists, proton pump inhibitors (PPIs), and antacids should be avoided.36–39 The ATV trough was decreased by 78% and 28% with omeprazole and famotidine co-administration, respectively. The interaction with PPIs cannot be overcome with higher ATV doses, dose separation, or “boosting” with RTV. If patients require acid suppression, H-2 blockers can be given 2 h after or 10 h before unboosted ATV administration; alternatively, “boosting” with RTV can minimize the decrease in ATV serum level observed with H-2 blockers.38,39 Fluconazole or voriconazole are an appropriate alternative in patients requiring agents that raise gastric pH, or in those with achlorhydria, because their absorption is not dependent on gastric pH.40,41 Alternatively, ketoconazole or itraconazole can be given with an acidic beverage such as Coca-Cola® to improve absorption, but this approach was not effective in reversing ATV-omeprazole interaction.38,42
Altered Drug Metabolism
Intestinal metabolism and P-glycoprotein
The cytochrome P450 (CYP) enzyme system consists of at least 12 families of enzymes common to all mammals, and represents the major enzyme system involved in drug metabolism.43 In humans, the CYP1, CYP2, and CYP3 families are primarily responsible for drug metabolism, with the CYP3A subfamily involved in the metabolism of the largest number of drugs, including most available PIs and non-nucleoside reverse transcriptase inhibitors (NNRTIs). CYP-mediated metabolism largely takes place in the liver, although CYP enzymes are also present in other sites including enterocytes in the intestinal wall.44 Thus, inhibitors of CYP3A4 may alter both drug absorption and hepatic metabolism. The 20-fold increase in plasma concentrations of SQV produced by RTV is likely caused by inhibition of CYP3A4 at both sites.45 Grapefruit juice contains various substances that inhibit CYP3A4-mediated metabolism only in the gut wall, mainly by selective down regulation of CYP3A4 activity in the small intestine.46 The AUC for SQV is increased by 1.5- to 2.5-fold during concomitant administration with grapefruit juice.24 However, grapefruit juice should not be relied upon to increase the plasma concentrations of PIs because the amounts of inhibitors vary widely between brands and are affected by factors such as how much and how often the juice is consumed.47
P-glycoprotein (P-gp) is the product of the mdr1 gene first described as a mediator of resistance to cancer chemotherapy. Enterocytes in the intestinal mucosa are a major site for expression of P-gp, one of several membrane-bound proteins that increase efflux of drugs from cells.48 P-gp appears to contribute to the low bioavailability of some drugs, including certain PIs. P-gp in the brush border cells of the intestine can pump drug back into the gastrointestinal lumen, decreasing absorption. In the liver, P-gp pumps drug into bile; it is also present in the blood–brain barrier, where it can limit the uptake of drugs into the central nervous system. Several PIs are substrates for and inhibitors of P-gp. In theory, inhibition of P-gp could be used to increase PI concentrations in target sites such as the CNS.49 However, P-gp expression has a profound negative impact on HIV replication. In two separate sets of experiments, cells expressing P-gp produced at least 40 to 70-fold less HIV than control cells. This was thought to be primarily due to inhibition of HIV entry and/or membrane fusion.50,51 The clinical implications of these findings highlight a dilemma regarding how (or whether) P-gp should be altered in HIV-infected patients: the presence of P-gp may lead to decreased intracellular drug concentrations, but the fact that P-gp expression may make cells less susceptible to HIV infection might counteract these low drug concentrations. The clinical relevance of P-gp for antiretriovirals remains to be determined.
CYP enyzmes and P-gp in the intestine and liver can present a barrier to absorption of orally administered drugs, and have considerable impact on drug interactions. The overlap of tissue distribution and substrate specificity of CYP3A4 and P-gp complicates definition of the specific mechanisms of some drug interactions. Many drugs that are modulators of P-gp are also inhibitors of CYP3A4.52 The effect of these two pathways on antiretroviral drug concentrations remains a fertile area for additional research.
Enzyme Induction
Inducers increase the rate of hepatic metabolism, usually through increased transcription of mRNA, and decrease serum concentrations of other drugs metabolized by the same hepatic isoenzyme. Rifampin and rifabutin are classic examples of enzyme inducers, and cause decreases in plasma concentrations of concomitant metabolized drugs. Both drugs can decrease concentrations of all HIV PIs. The Centers for Disease Control and Prevention (CDC) have issued guidelines for concomitant use of rifampin or rifabutin with HIV PIs in patients with tuberculosis.53 Rifampin should be avoided with most PIs and NNRTIs but may be used with a higher efavirenz (EFV) dose (800 mg).53 RTV with SQV should be avoided with rifampin co-administration due to a high incidence of hepatitis.54 Although, the addition of extra RTV (300 mg bid) to LPV counteracted rifampin’s enzyme induction, this strategy is limited due to a high incidence of undesirable side effect.55 Patients receiving IDV or nelfinavir should receive a reduced dose of rifabutin and increased PI dose.53,56
Nevirapine is a mild to moderate hepatic enzyme inducer, and decreases the AUC of LPV, IDV, and SQV by 22–38%, but has a minimal effect on RTV and nelfinavir.10,57,58 Boosting PIs with RTV or increasing the PI dose (i.e., LPV/r to 600/150 mg bid) can counteract the inducing effect of nevirapine.10 EFV is a mixed inducer/inhibitor in vitro; however, in vivo a decrease in concentrations of FPV, ATV, LPV/r, SQV, and IDV is observed with co-administration. Increasing LPV/r dose to 600/150 mg twice-daily or the addition of RTV to ATV, FPV, and SQV is required with EFV co-administration.59
RTV and nelfinavir are also moderate enzyme inducers, and can increase hepatic glucuronidation as well as CYP activity. The AUC of the oral contraceptive ethinyl estradiol is decreased by ∼40% with these agents, necessitating an alternative form of birth control.7,60 Conversely, IDV and ATV are inhibitors of hepatic glucuronidation which resulted in a 24% and 48% increase in AUC of ethinyl estradiol, respectively.26,61 RTV is also an inducer of CYP1A2 which is involved in the metabolism of theophylline and olanzapine.62.63 Concomitant administration of RTV with theophylline or olanzapine has been reported to result in a decrease in the theophylline AUC of 43% and olanzapine AUC of 53%. Patients receiving these combinations should be monitored for reduced efficacy and the dose should be titrated to therapeutic effect.
TPV is a nonpeptidic HIV PI. In vivo, TPV is a substrate and potent inducer of the P-gp drug transporter (P-gp). In vitro metabolism studies with human liver microsomes also indicate that TPV is a substrate of CYP3A4.64 RTV, a known inhibitor of CYP3A4 and P-gp, significantly decreases TPV clearance, resulting in a 29-fold increase in the TPV AUC.64a TPV is therefore dependent on RTV co-administration to achieve the desired pharmacokinetic parameters. TPV/r (500/200 mg BID) produces a net inhibition of CYP3A4, resulting in an increase in plasma concentrations of many CYP3A4 substrates (e.g., atorvastatin). However, for drugs that are dual substrates of CYP 3A4 and P-gp, a net induction results in a 45%, 49%, and 70% reduction in the amprenavir (APV), LPV (LPV/r), and SQV AUCs, respectively.64b
Enzyme Inhibition
There are a number of inhibitors of CYP that decrease the rate of hepatic metabolism and increase plasma concentrations of other drugs metabolized by the same isoenzyme. HIV PIs can be both CYP inhibitors and substrates, increasing concentrations of some metabolized drugs and having their own concentrations increased by other CYP inhibitors. With the exception of nelfinavir, all of the currently available PIs are primarily metabolized by CYP3A4.5 These agents differ in the number and magnitude of potential drug interactions. RTV is associated with the greatest number of drug interactions, while SQV is the weakest enzyme inhibitor, and has less propensity to alter concentrations of other drugs. FPV, nelfinavir, and IDV inhibit CYP3A4 metabolism to a lesser extent than RTV. For example, all three drugs increase the rifabutin AUC approximately twofold, necessitating a 50% reduction in the rifabutin dose.19 RTV increases the rifabutin AUC fourfold, which may lead to clinically significant adverse effects when clinicians did not reduce dose of rifabutin.65,66 Since most PIs are co-administered with RTV, they have a similar drug interaction profile to that of RTV. However, there are some exceptions to this rule. For example, enzyme induction predominates when FPV and LPV/r are co-administered; this interaction resulted in a 64% and 48% decrease in amprenavir and LPV AUC, respectively.67 Higher doses of both FPV and LPV only partially counteracted the observed interaction.68 Similarly, when TPV is given with amprenavir/r, LPV/r, or SQV/r, a 45–70% decrease in AUC of the co-administered PI was observed.69 Conversely, the clarithromycin AUC is increased 53% with IDV, 77% with RTV, and 94% with ATV. Clarithromycin dosage adjustment with most PIs is unnecessary in patients with normal renal function; however, a 50% dose reduction is recommended with ATV co-administration due to an observed QTc prolongation.8,70 Azithromycin is primarily excreted by the biliary route and does not interact with inhibitors of CYP; therefore, it is a good alternative to clarithromycin.71
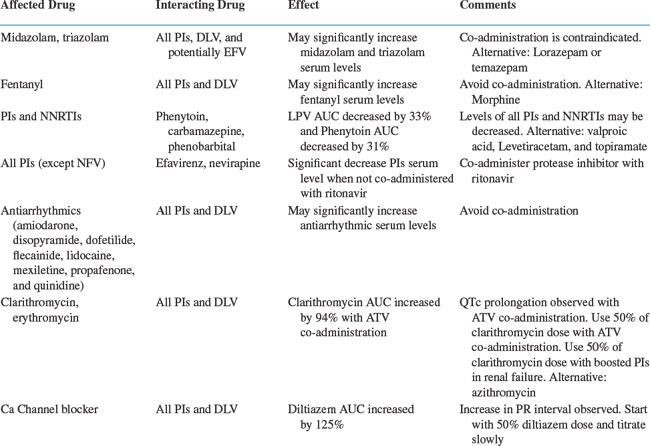
Life-Threatening Drug Interactions
The most serious potential interactions with CYP inhibitors involve concomitant administration with certain metabolized drugs such as terfenadine and cisapride (both removed from the US market), antiarrhythmics, and ergot alkaloids, which all have a low therapeutic index. In the case of cisapride and terfenadine, these combinations led to cardiotoxicity, with the potential for life-threatening arrhythmias.72 Administration of PIs with some benzodiazepine sedative-hypnotics (i.e., midazolam and triazolam) and certain opiates (i.e., fentanyl) can result in exaggerated side effects such as oversedation and possible respiratory depression.73,74
Beneficial Drug Interactions
Drug interactions were initially viewed as a complication to be avoided in HIV-infected patients. The concept of using two PIs concomitantly to increase or “boost” plasma concentrations or improve convenience was first recognized with the combination of SQV and RTV. Simultaneous administration of two PIs takes advantage of beneficial pharmacokinetic interactions, and may circumvent many of the drugs’ undesirable pharmacologic properties.6 In addition, dual PIs decrease interpatient variability, making drug concentrations more predictable. Dual PI regimens have now been widely prescribed and are the standard of care in published treatment guidelines.75 A number of potentially beneficial metabolic drug interactions exist for combinations of two HIV PIs. One drug, generally RTV, is used to inhibit the metabolism of the second agent, producing increased bioavailability, decreased clearance, or both. Two-, three-, and four-way interactions are no longer uncommon, in which case the pharmacokinetic profile of each drug can be benefited or adversely affected.
RTV-Boosted PI Combinations
Saquinavir-RITONAVIR
When SQV was first introduced, it possessed a number of disadvantages including poor bioavailability, three times daily dosing, and a pill burden of 18 capsules per day. However, when combined with even small doses of RTV, there is a marked increase in SQV bioavailability and a decrease in clearance, which allows twice-daily dosing with a lower pill burden (six per day). In a single-dose, crossover study in healthy volunteers, RTV increased the SQV AUC by 50- to 132-fold, and increased the SQV Cmax by 23- to 35-fold.45 Since RTV is a P450 inducer and undergoes autoinduction during the first 10–14 days of therapy,76 steady-state concentrations of SQV should be lower when these two drugs are combined. Multiple-dose pharmacokinetic interaction studies found that the steady-state SQV AUC was increased only 20- to 30-fold.77
The effect of RTV on SQV plasma concentrations was evaluated in 120 patients receiving various SQV/r combinations.78 Data from two dose-ranging trials of SQV/r given either twice daily or once daily were included in the analysis. A wide range of SQV doses (400–1800 mg) and RTV doses (100–400 mg) were evaluated. The investigators used multivariate linear and nonlinear regression to correlate steady-state SQV pharmacokinetic parameters (Cmin, Cmax, and half-life) with SQV and RTV doses. This model showed a strong effect of RTV on SQV Cmax and Cmin; however, these parameters were correlated only with SQV dosage, and the increase in SQV concentrations was similar for RTV dosages over the range of 100–400 mg twice daily. In this analysis there was no dose-dependent effect of RTV on SQV concentrations.
The poor oral bioavailability of SQV (1–12%, depending on formulation and conditions) likely reflects extensive first-pass metabolism rather than poor absorption. The increase in SQV concentrations with RTV is the result of improved bioavailability, perhaps to as much as 100%, with little effect on postabsorptive systemic clearance. In addition to effects on intestinal CYP 3A4, RTV is also an inhibitor of P-gp and its dual effects on both CYP3A4 and P-gp may account for the large magnitude of RTV’s effect on SQV’s oral bioavailability.79
Although the dose of 400 mg ritonavir/400 mg SQV twice daily was initially used, the high rate of GI intolerance, hepatitis, and hyperlipidemia led to “boosting” SQV with lower doses of RTV. In an open-label pharmacokinetic study, three SQV/r regimens (SQV/r 1600/100 mg once daily, 2000/100 mg once daily, and 1000/100 mg twice daily) were evaluated. SQV/r 2000/100 mg once daily resulted in a 71% higher AUC compared to SQV/r 1600/100 mg once daily. The AUC was comparable between SQV 1000/100 mg twice daily and SQV 2000/100 mg once daily, but the twice-daily arm achieved a significantly higher Cmin.45 Clinically, SQV/r 1000/100 mg bid was compared to LPV/r (400/100 mg bid), both with more than two NRTI/NNRTIs, in 324 HIV-infected patients. The population studied was heterogeneous and included 33% antiretroviral-naive, 48% PI-naive, and 32% who had experienced virologic failure with more than one PI. Risk of virologic failure was higher in the SQV/r arm than the LPV/r arm by intent-to-treat analysis.80 In another trial, SQV/r 1600/100 mg qd also underperformed compared to EFV with NRTIs in 161 antiretroviral-naive patients. At 48 wks, viral suppression to below 50 copies/mL was 71% versus 51%, in the EFV and SQV/r arms, respectively.81
RTV-IDV
When used as a sole PI, IDV possesses a number of limitations including an every 8-h dosing regimen, food restrictions, hydration requirements, and large interindividual pharmacokinetic variability.5 The combination of IDV with RTV alleviates many of these disadvantages.
In a steady-state pharmacokinetic interaction study in healthy volunteers involving 14 days of RTV treatment, the combination of 200 or 400 mg RTV with 400 or 600 mg IDV increased the IDV AUC by three- to sixfold compared to IDV 800 mg alone.82 This mechanism primarily involves inhibition of hepatic CYP 3A4 with reduced first-pass metabolism making a minor contribution. RTV increased the IDV Cmax up to twofold, and increased the IDV concentration 8 h after dosing by 11- to 33-fold.82 Decreased RTV dose and increased IDV dose were examined in a separate study.83 In healthy volunteers administered RTV for 14 days, the 24-h IDV AUC with a 800 mg bid IDV/100 mg bid RTV regimen was fourfold higher than with 800 mg q8h IDV alone. In the same study, the 24-h AUC of IDV with a 400/400 bid RTV-IDV regimen was 40% lower than with the 800/100 bid regimen and 55% lower than with a 800/200 bid regimen.83 However, the mean 12-h trough concentrations of the 400/400 bid regimen and the 800/100 bid regimen were nearly the same. The 800/100 bid regimen is generally recommended due to the lower rate of GI intolerance, but if EFV or nevirapine is co-administered with IDV/RTV, the 800/200 mg bid regimen is recommended since EFV decreases the IDV AUC by 30%.84
In two studies, co-administration of RTV and IDV abolished the effect of food on IDV bioavailability. A high-fat meal reduced the bioavailability of unboosted IDV by up to 85%.82 Doses of 100, 200, or 400 mg of RTV bid reversed the effect of a high- or low-fat meal on IDV pharmacokinetics, as compared to 800 mg IDV given in the fasted state.83 RTV could enhance IDV oral bioavailability in the presence of food through inhibition of intestinal CYP or drug transporters such as P-gp. This finding suggests that the deleterious effects of food on IDV may be mediated by interaction with intestinal epithelial drug transporters or P450 complexes, processes potentially blocked by RTV.6
There is some concern that the high concentrations achieved with IDV/RTV may lead to an increased risk of nephrolithiasis. In patients taking IDV 800 mg q8h, a higher AUC and Cmax were associated with increased risk of IDV nephrotoxicity in one study.85 Increasing IDV Cmax and Cmin were associated with an increase in nephrolithiasis in patients taking IDV/RTV bid combinations of 400/100, 400/400, 600/100, or 800/100 mg; these regimens were associated with short-term nephrotoxicity risks of 0%, 2%, 6%, or 10%, respectively.86 IDV/RTV regimens of 400/400 mg bid were not associated with an increased incidence of nephrolithiasis compared to IDV 800 mg q8h without RTV, although tolerability may be an issue with the higher RTV dose.87
Lopinavir-Ritonavir
LPV-RTV (Kaletra®) is the only co-formulated product designed to increase concentrations of a second PI by drug interaction. Each dose of Kaletra® contains 400 mg of LPV along with 100 mg of RTV (200/50 mg tablets 3 2 tablets) which is given twice daily. LPV is a highly active PI but its bioavailability is low and its clearance rapid when given alone. However, in combination with low doses of RTV, the AUC is increased by greater than 100-fold,88 due to inhibition of LPV’s CYP3A4 metabolism in the liver and gastrointestinal tract. RTV’s effect on the LPV AUC was several-fold greater than RTV’s effect on the SQV AUC.6
In human volunteers mean trough concentrations of LPV were ∼30-fold higher than the in vitro IC50 for HIV.89 In 101 HIV-infected patients taking LPV 400 mg bid with RTV 100 mg bid with two nucleoside analogs for 48 weeks, HIV viral load (VL) was suppressed to less than 400 copies/mL in 93–100% of patients and to below 50 copies/mL in 83–86%.90
The addition of EFV to LPV-RTV results in a decrease of 40% in the LPV AUC.91 Although concentrations are still relatively high despite this reduction, the manufacturer suggests the dose be increased to 600/150 mg (three tablets) bid when combined with EFV or nevirapine in order to offset the enzyme induction effects of the NNRTIs, especially if the patient is treatment-experienced and resistance is suspected.10
Fosamprenavir-Ritonavir
Administration of FPV with low-dose RTV increases the amprenavir AUC twofold and increases the trough concentration by approximately sixfold compared to FPV without RTV.11 The current dosing recommendation is FPV/RTV 700/100 mg bid or 1400/200 mg once daily, but once-daily administration should be reserved for PI-naive patients due to lower amprenavir trough concentrations compared to twice-daily administration.11
In patients also receiving nevirapine or EFV, 100 mg bid of RTV does not appear to prevent a decrease in amprenavir concentrations (when given as the amprenavir capsule). In a small study, patients receiving 450 mg of amprenavir with 200 mg bid of RTV were switched to a 600/100 mg regimen and amprenavir trough values were decreased by 80%.92 However, there was only a 17% decrease in amprenavir’s trough when FPV/RTV 700/100 mg bid is co-administered with EFV.11 EFV also decreased the IDV AUC by 30% when added to IDV/RTV 800/100 mg bid.84 Thus, there appears to be a threshold dose of at least 200 mg of RTV that is required to prevent NNRTI-induced drug interactions for some PIs (i.e., amprenavir and IDV) but not others (i.e., ATV and FPV). The RTV dose should therefore be increased to 200 mg bid in IDV/RTV or amprenavir/RTV combinations with EFV or nevirapine.10
Clinically, FPV/RTV 700/100 mg bid, FPV/RTV 1400/200 mg qd, and LPV/RTV were compared in an open-label, randomized trial involving 315 PI-experienced patients. VL less than 50 through wk 48 was observed in 37%, 46%, and 50% receiving qd FPV, bid FPV, and LPV/RTV, respectively. The lower amprenavir trough may have contributed to the worse performance seen with the daily FPV regimen, and this regimen is not recommended for PI-experienced patients.93
RTV–Nelfinavir
Originally marketed as a thrice-daily drug, nelfinavir was studied in combination with RTV to reduce dosing frequency and evaluate the potential for once-a-day dosing. While RTV does increase nelfinavir concentrations modestly, overall, the AUC of nelfinavir was increased by only 30%. This combination is generally not widely used due to poor tolerability with only marginal pharmacokinetic benefit.94 Dose limiting diarrhea or gastrointestinal adverse effects make this regimen less attractive than other dual PI combinations.95
Nelfinavir is the only HIV PI known to produce an active metabolite, the hydroxy-butylamide M8 (AG1402), which is the major metabolite of nelfinavir in humans and has equipotent anti-HIV activity in vitro.96 RTV had a more significant beneficial impact on the pharmacokinetics of M8 than on nelfinavir itself. After 5 weeks of dosing, RTV use was associated with a 430% increase in the 500 mg M8 24-h AUC, and a 370% increase in the 750 mg M8 AUC, as compared to historical controls taking nelfinavir 750 mg tid alone.6
Atazanavir-Ritonavir
ATV is the first PI that is not dependent on RTV for once-daily dosing. However, in antiretroviral-experienced patients, unboosted ATV 400 mg qd is inferior to LPV/RTV (LPV/r 400/100 mg bid).97 In an effort to improve the pharmacokinetic parameters of ATV, ATV 300 mg plus RTV 100 mg was evaluated. The ATV AUC and Cmin increased twofold and 10-fold, respectively.8 In a subsequent study involving antiretroviral-experienced patients, boosted ATV (ATV/r 300/100 mg qd) was compared to LPV/r 400/100 mg bid At 48 wks, 56% versus 58% achieved VL below 400 in ATV/r and LPV/r arm, respectively.98 Thus, the beneficial pharmacokinetic interaction of boosted ATV resulted in improved clinical outcome in PI-experienced patients.
TPV-RTV
The pharmacokinetics of TPV, a nonpeptidic PI, are greatly improved by RTV. In an open-label, randomized, parallel-group trial involving 95 healthy subjects, RTV increased the TPV Cmin 20-fold. This increase allows the TPV average steady-state Cmin to be 20–57 times the protein-adjusted IC90 for PI-resistant HIV-1.99 The potency of TPV/RTV (TPV/r) 500/200 mg bid in treatment-experienced patients with VL above 1000 copies/mL and more than one primary PI mutation was demonstrated in the RESIST I trial. At 24 wks, 34.7% in the TPV/r group and 16.5% of participants in the comparator PI group achieved a VL below 400 copies/mL.100
Dual-Boosted PI Combinations
Lopinavir/Ritonavir (LPV/r)-Atazanavir (ATV)
In a crossover pharmacokinetic study, the combination of ATV 300 mg qam with LPV/RTV (LPV/r) 400/100 mg bid resulted in a 45% higher ATV Cmin compared to ATV/r 300/100 mg qd; however, ATV AUC0–24 and Cmin were not significantly different. Additional boosting of ATV with RTV 100 mg qd when co-administered with LPV/r did not further increase the ATV Cmin but did increase the LPV Cmin by 28%. LPV PK parameters were comparable to historical data from volunteers receiving LPV/r 400/100 mg bid, at steady state, without ATV co-administration.101 In several retrospective analyses involving both PI-experienced and naive patients, ATV with LPV/r combination as part of an antiretroviral regimen resulted in an undetectable VL in 77–97% of treated patients.102–104 In a small pilot study in 12 patients who had failed at least two different PI-containing regimens, virologic suppression was reported in six of the 12 patients treated with ATV and LPV/r as part of their antiretroviral regimen.105
Lopinavir/ritonavir-Fosamprenavir
The combination of fosamprenavir (FPV) and lopinavir-ritonavir (LPV/r) may be a good option in treatment-experienced patients due to the nonoverlapping resistance mutations.106 AIDS Clinical Trials Group (ACTG) Protocol 5143 was a randomized study that compared single-boosted PI regimens (LPV/r 400/100 mg bid or FPV/r 700/100 mg bid) to a dual-PI regimen (LPV/r 400/100 mg bid 1 FPV 700 mg bid) in combination with tenofovir DF and nucleosides in highly treatment-experienced patients.107,108 Due to an adverse drug interaction in the dual-PI arm that resulted in a 55% and 60% decrease in the LPV and APV median AUC0–12, respectively, study enrolment was stopped early after only 56 subjects out of a planned sample size of 216. Despite this interaction, at week 24, the dual-PI regimen had a comparable virologic response to the single-PI regimen, with 75% (n = 28) and 61% (n = 28) of patients having a greater than 1.0 log decline in VL from baseline with the dual-PI and single-PI regimens, respectively (intent-to-treat, P =0.17). HIV-1 RNA was below 50 copies/mL in 54% and 46% of double and single PI subjects, respectively (intent-to-treat, P =0.37).107 The result of this study should be interpreted with caution, since the study was underpowered to detect a potential difference between the two groups.
Lopinavir/ritonavir-Saquinavir
The effect of lopinavir/ritonavir (LPV/r) on saquinavir (SQV) plasma concentrations was evaluated in 45 patients receiving LPV/r 400/100 mg bid and SQV 1000 mg bid. SQV exposure with LPV/r was not significantly lower compared to patients receiving SQV/r 1000/100 mg bid in the control arm, even though the RTV AUC was 55% lower. LPV concentrations were similar to historical control data.109 The LPV with SQV combination was evaluated in a cohort study of 126 heavily pretreated patients who were experiencing treatment failure with nucleoside reverse transcriptase inhibitor (NRTI)-containing regimen. At week 48, a switch to LPV/r 400/100 mg with SQV 1000 mg bid without the addition of RTIs resulted in a VL decrease by a median 2.7 log10.110
RTV-Sparing Dual PI Combinations
A high incidence of hyperlipidemia and GI intolerance associated with RTV boosted PIs combination have led to the evaluation of dual PI combinations without RTV. Combining nelfinavir 750 mg tid with 800 mg tid of the soft-gel formulation of SQV produced an SQV AUC equivalent to 1200 mg tid at steady state.111 The substitution of SQV soft-gel capsule formulation with the commercially available formulation (SQV hard-gel capsules or tablets) will likely result in a similar pharmacokinetic profile, but this combination is less relevant to clinical practice due to the large pill burden and thrice-daily administration. IDV and SQV combinations, when dosed twice-daily at steady state had a disappointing pharmacokinetic enhancement profile, this led to only 45% of patients suppressing their plasma HIV RNA to less than 400 copies/mL after 72 weeks.112 The IDV/SQV combination was reported to be antagonistic when used to inhibit HIV replication in vitro.113 While the clinical relevance of this finding is unknown, this combination has not been evaluated in clinical studies and should be avoided.
The combination of FPV and ATV was evaluated in a prospective, randomized, open-label, three-way crossover pharmacokinetic study, in which 21 HIV-infected adults received FPV 1400 mg, ATV 400 mg, or both once-daily for 14 days. Although the AUC of FPV was increased by 78%, ATV AUC and Cmin were decreased by 33% and 57%, respectively.114 This combination is not recommended since ATV Ctrough falls below the range of 150–850 ng/mL which has been associated with the highest probability of virologic response.115
A small pilot study examined the pharmacokinetics of ATV 400 mg qd when given alone compared to when given in combination with SQV 1200 mg hard-gel capsules in 22 HIV-infected patients. Although the SQV AUC was increased threefold, the ATV pharmacokinetic profile was not enhanced by SQV co-administration.8,116 The lack of a beneficial pharmacokinetic interaction between ATV 400 mg qd and SQV 1200 mg qd may partially explain the lower virologic response rate compared to ATV/r 300/100 mg qd or LPV/r 400/100 mg bid in treatment-experienced patients.98 Numerous attempts to boost PI concentrations without RTV have fallen short in achieving the desired serum level needed to treat PI-experienced patients.
Other Antiretroviral Combinations
Other strategies for increasing concentrations of PIs through enzyme inhibition could include concomitant ketoconazole or delavirdine, both of which are CYP3A4 inhibitors. Delavirdine significantly increased the AUC of SQV by 520%, IDV by 72%, and nelfinavir by 92%.117,118 However, due to the lack of clinical data and weak antiviral potency of delavirdine, these combinations are not recommended as initial therapy.75
Interactions with Herbal Therapies
Herbal remedies and nutritional supplements are widely used in HIV-infected patients although little attention has been paid to the pharmacokinetic effects of these compounds since they are considered benign. An increasing number of studies have shown that certain alternative therapies may cause drug interactions with agents used in the treatment of HIV infection. In healthy volunteers, St John’s wort decreased the AUC of IDV by over 50%.119 The mechanism of this interaction is complex and appears to be mediated by both induction of CYP3A4 and P-gp.120,121 This herb should be avoided in patients taking PIs and NNRTIs, although there currently are no data on whether RTV can reverse this interaction.
Garlic supplements are sometimes used by HIV-infected patients because of their touted effects on lowering cholesterol. Raw garlic and garlic supplements inhibit the activity of CYP3A4 in vitro and in animals, and case reports have documented RTV-related gastrointestinal toxicity in two people after they ingested uncooked garlic preparations with RTV.122 However, a study in healthy volunteers showed that garlic capsules taken bid for 3 weeks led to a mean decrease in SQV concentrations of ∼50% probably as a consequence of reduced bioavailibility.123 Even after a 10-day washout period, AUC values returned to only 60–70% of baseline suggesting a prolonged effect. Other herbs with reported in vitro effects on CYP450-mediated metabolism include silymarin (milk thistle), ginseng, and skullcap.124 IDV Cmin was decreased by 25% with milk thistle co-administration, but the AUC remained unchanged.125 Clinicians need to include alternative medicines in their drug histories and consider them when adverse effects or treatment failure appear with no other cause.
Drug–Cytokine Interactions
Proinflammatory cytokines including interleukin (IL)-6, IL-1, and tumor necrosis factor (TNF)-a are released during periods of stress, trauma, or infection. A number of in vitro and clinical studies have shown that IL-6 and TNF-α inhibit CYP-mediated metabolism. This mechanism is not competitive but is a metabolic interaction at the level of transcription of CYP messenger RNA.126
Several immunodulating agents are being evaluated for the treatment of HIV-infection. One of the best studied is IL-2. Its exogenous administration has been shown to increase CD4+ T-lymphocyte cells, but results in a profound release of proinflammatory cytokines that are one likely cause of its problematic side effect profile. In HIV-infected patients receiving a 5-day continuous infusion of IL-2, IDV clearance significantly decreased and the AUC increased 75% as compared with baseline values before IL-2 administration.127 The short-term administration (5 days) of IL-2 makes this interaction less clinically significant, although drug–cytokine interactions should be considered as additional investigational agents are used in a more chronic fashion.
Altered Excretion
Drug interactions may also be caused by alterations in renal elimination. This can be a consequence of either inhibition of tubular secretion or impairment of renal function. Probenecid and trimethoprim are inhibitors of renal tubular secretion, which may increase concentrations of some renally cleared drugs. The lamivudine AUC is increased by 44% with concomitant trimethoprim-sulfamethoxazole.128 The acyclovir AUC is increased 40% with concomitant probenecid.129 Inhibition of renal secretion with probenecid is a useful strategy to increase plasma concentrations of procaine penicillin, needed for the treatment of neurosyphilis.130 tenofovir DF (TDF) clearance decreased by 16% with LPV/r co-administration The mechanism for this interaction is unknown. Although this interaction is not likely to be clinically significant, there have been several cases of nephrotoxicity when these two agents are co-administered.131,132
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree