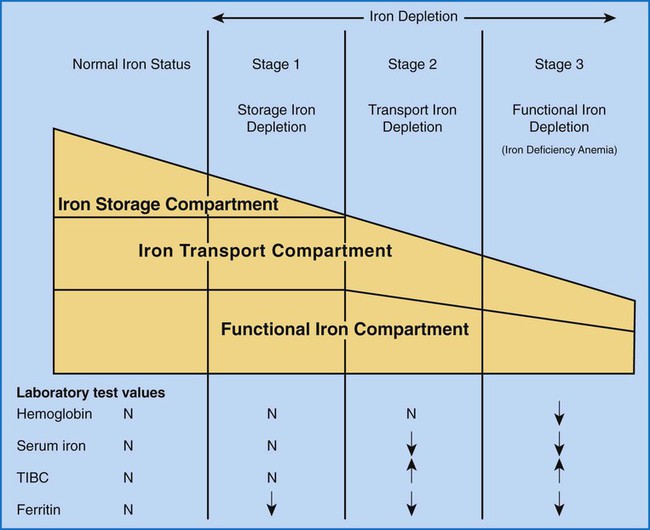
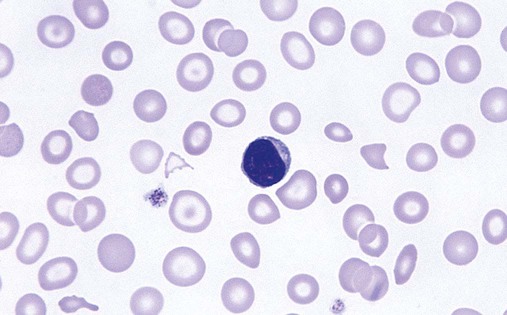
After completion of this chapter, the reader should be able to: 1. Recognize complete blood count (CBC) results consistent with iron deficiency anemia, anemia of chronic inflammation, and sideroblastic anemias. 2. Given the results of iron studies, including free erythrocyte protoporphyrin (FEP), serum transferrin receptor, and reticulocyte hemoglobin, distinguish findings consistent with iron deficiency anemia, anemia of chronic inflammation, sideroblastic anemias, thalassemias, and iron overload conditions. 3. Recognize individuals at risk for iron deficiency anemia by virtue of age, gender, diet, physiologic circumstance such as pregnancy and menstruation, or pathologic conditions such as gastric ulcers. 4. Given a description of the appearance of bone marrow stained with Prussian blue stain, recognize results consistent with iron deficiency anemia, anemia of chronic inflammation, or a sideroblastic anemia. 5. Recognize a bone marrow description consistent with iron deficiency anemia, anemia of chronic inflammation, or a sideroblastic anemia. 6. Recognize conditions in which anemia of chronic inflammation may develop. 7. Recognize predisposing factors for sideroblastic anemias or conditions in which sideroblastic anemias may develop. 8. Discuss the clinical significance of increased levels of FEP. 9. Describe the pathogenesis of iron deficiency anemia, anemia of chronic inflammation, sideroblastic anemia secondary to lead poisoning, and hemochromatosis. 10. Discuss the differences in disease etiology, diagnosis, and treatment between iron overload resulting from hereditary hemochromatosis and transfusion-related hemosiderosis. 1. What conditions should you consider based on the results of the CBC? 2. Assuming that this patient has not been diagnosed with anemia at any other time during her life, are any of the conditions listed in the answer to question 1 more likely, based on the patient’s age or sex? 3. Assuming that the patient is otherwise healthy and experiencing only the common declines of sight, hearing, and mobility associated with aging, are any of the conditions listed in the answer to question 1 more likely than the others? 4. What additional testing would you recommend? What results do you expect for this patient? Anemia may result whenever red blood cell (RBC) production is impaired, RBC life span is shortened, or there is frank loss of cells. The anemias associated with iron and heme typically are categorized as anemias of impaired production resulting from the lack of raw materials for hemoglobin assembly. Depending on the cause, lack of available iron results in iron deficiency anemia or the anemia of chronic inflammation. Inadequate production of protoporphyrin leads to diminished production of heme and thus hemoglobin, but with a relative excess of iron. The result is sideroblastic anemia. These causes are discussed in this chapter. Inadequate globin production results in the thalassemias, which are discussed separately in Chapter 27. As discussed more extensively in Chapter 11, iron is absorbed from the diet in the small intestine, carried by transferrin to a cell in need, and brought into the cell, where it is held as ferritin until incorporated into its final functional molecule. That functional molecule may be a heme-based cytochrome, muscle myoglobin, or, in the case of developing RBCs, hemoglobin. Iron may be unavailable for incorporation into heme because of inadequate stores of body iron or impaired mobilization. The anemia associated with inadequate stores is termed iron deficiency anemia, whereas the anemia resulting from impaired iron mobilization is known as anemia of chronic inflammation because of its association with chronic inflammatory conditions, such as rheumatoid arthritis. When the iron supply is adequate and mobilization is unimpaired but an intrinsic RBC defect prevents production of protoporphyrin or incorporation of iron into it, the resulting anemia is termed sideroblastic, which refers to the presence of nonheme iron in the developing RBCs. Iron deficiency anemia can develop when the erythron is slowly starved for iron. Each day, approximately 1 mg of iron is lost from the body, mainly in the mitochondria of desquamated skin and sloughed intestinal epithelium.1 Because the body tenaciously conserves all other iron from senescent cells, including RBCs, daily replacement of 1 mg of iron from the diet maintains iron balance and supplies the body’s need for RBC production as long as there is no other source of loss. When the iron in the diet is consistently inadequate, over time the body’s stores of iron become depleted. Ultimately, RBC production slows as a result of the inability to produce hemoglobin. With approximately 1% of cells dying naturally each day, the anemia becomes apparent when the production rate is insufficient for replacement of lost cells. Iron deficiency anemia develops slowly, progressing through stages that physiologically blend into one another but are useful delineations for understanding disease progression.2 As shown in Figure 19-1, iron is distributed among three compartments: (1) the storage compartment, principally as ferritin in the bone marrow macrophages and liver cells; (2) the transport compartment of serum transferrin; and (3) the functional compartment of hemoglobin, myoglobin, and cytochromes. Hemoglobin and intracellular ferritin constitute nearly 95% of the total distribution of iron.3 Stage 1 of iron deficiency is characterized by a progressive loss of storage iron. RBC development is normal, however, because the body’s reserve of iron is sufficient to maintain the transport and functional compartments through this phase. There is no evidence of iron deficiency in the peripheral blood because RBCs survive 120 days, and the patient does not experience symptoms of anemia. Serum ferritin levels are low, however, which indicates the decline in stored iron, and this also could be detected in an iron stain of the bone marrow. Without evidence of anemia, however, neither of these tests would be performed, and individuals appear healthy. The prevalence of stage 1 iron deficiency in the United States has been estimated at 14.4% for 1- to 2-year-olds, 3.7% for 3- to 5-year-olds, 9.3% for 12- to 19-year-old females, and 9.2% for 20- to 49-year-old females.4 Stage 3 of iron deficiency is frank anemia. The hemoglobin concentration and hematocrit are low relative to the reference ranges. Because of the thoroughly depleted storage iron and diminished transport iron, RBC precursors are unable to develop normally. The number of cell divisions per precursor increases because hemoglobin accumulation in the developing cells is slowed, which allows more time for divisions. The result is first smaller cells with adequate hemoglobin concentration, although ultimately even these cannot be filled with hemoglobin. The RBCs then become microcytic and hypochromic (Figure 19-2). As expected, serum ferritin levels are exceedingly low. Results of other iron studies (see later) are also abnormal, and the FEP and transferrin receptor levels continue to increase. In this phase, the patient experiences the nonspecific symptoms of anemia, typically fatigue and weakness, especially with exertion. Pallor is evident in light-skinned individuals but also can be noted in the conjunctivae, mucous membranes, or palmar creases of dark-skinned individuals.3 More severe signs are not seen as often in the United States3 but include a sore tongue (glossitis) due to iron deficiency in the rapidly proliferating cells of the alimentary tract and inflamed cracks at the corners of the mouth (angular cheilosis). Koilonychia (spooning of the fingernails) may be seen if the deficiency is long-standing. Patients also may experience cravings for nonfood items, called pica. The cravings may be for things such as dirt, clay, laundry starch, or, most commonly, ice (craving for the latter is called pagophagia).3 As should be evident from this discussion, numerous individuals may be iron deficient while appearing healthy. Until late in stage 2, they may experience no symptoms at all and are unlikely to come to medical attention. Even in stage 3, frankly anemic patients may not seek medical care, because the body is able to compensate remarkably for slowly developing anemia (see Chapter 18), as in the patient in the case study at the beginning of this chapter. Because results of routine screening tests included in the CBC do not become abnormal until late in stage 2 or early in stage 3, most patients are not diagnosed until relatively late in the progression of the iron depletion. From the previous discussion, it is apparent that certain groups of individuals are more prone to develop iron deficiency anemia. Menstruating women are at especially high risk. Their monthly loss of blood increases their routine need for iron, which often is not met with the standard U.S. diet.3 For adolescent girls, this is compounded by increased iron needs associated with growth. If women of childbearing age do not receive proper iron supplementation, pregnancy and nursing can lead to a loss of nearly 900 mg of iron,5 which further depletes iron stores. Succeeding pregnancies can exacerbate the problem, leading to iron deficient fetuses.5 Growing children also are at high risk.5 Growth requires iron for the cytochromes of all new cells, myoglobin for new muscle cells, and hemoglobin in the additional RBCs needed to supply oxygen for a larger body. The increasing need for iron as the child grows can be coupled with dietary inadequacies, especially in circumstances of poverty or neglect. Cow’s milk is not a good source of iron, and infants need to be placed on iron-supplemented formula by about age 6 months, when their fetal stores of iron become depleted.3 This assumes that the infants were able to establish adequate iron stores by drawing iron from their mothers in utero. Even though breast milk is a better source of iron than cow’s milk,6 it is not a consistent source.7 Therefore, iron supplementation is also recommended for breastfed infants after 6 months of age.3 Soldiers and long-distance runners also can develop iron deficiency. “Marching anemia” develops when RBCs are hemolyzed by foot-pounding trauma and iron is lost as hemoglobin in the urine.8 The amount lost in the urine can be so little that it is not apparent on visual inspection. Iron deficiency can be readily diagnosed in later stages using routine tests. Detection in the early stages requires sophisticated tests, but individuals are unlikely to be referred for such studies because there is virtually no physiologic evidence of the declining iron state. Nevertheless, early iron deficiency might be suspected in an individual in a high-risk group, and appropriate testing can be ordered.8 The tests for iron deficiency can be grouped into three general categories: screening, diagnostic, and specialized. When iron deficient erythropoiesis is under way, the CBC results begin to show evidence of anisocytosis, microcytosis, and hypochromia (see Figure 19-2). The classic picture of iron deficiency anemia in stage 3 includes a decreased hemoglobin level. An RDW greater than 15% is expected and may precede the decrease in hemoglobin.9 For patients in high-risk groups, the elevated RDW can be an early and sensitive indicator of iron deficiency.10 As the hemoglobin level continues to fall, microcytosis and hypochromia become more prominent, with progressively declining values for mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC). The RBC count ultimately becomes decreased, as does the hematocrit. Polychromasia may be apparent early, although it is not a prominent finding. A low absolute reticulocyte count confirms a diminished rate of effective erythropoiesis, because this is a nonregenerative anemia.11 Poikilocytosis, including occasional target cells and elliptocytes, may be present, although no particular shape is characteristic or predominant. Thrombocytosis may be present, particularly if the iron deficiency results from chronic bleeding, but this is not a diagnostic parameter. White blood cells (WBCs) are typically normal in number and appearance. Iron deficiency should be suspected when the CBC findings show a hypochromic, microcytic anemia with an elevated RDW but no consistent shape changes to the RBCs. Iron studies remain the backbone for diagnosis of iron deficiency (see Chapter 11). They include assays of serum iron, TIBC, transferrin saturation, and serum ferritin. Serum iron is a measure of the amount of iron bound to transferrin (transport protein) in the serum. TIBC is an indirect measure of transferrin and the available binding sites for iron in the plasma. The percent of transferrin saturated with iron can be calculated from the total iron and the TIBC: Ferritin is not truly an extracellular protein, because it provides an intracellular storage repository for metabolically active iron. However, ferritin is present in serum, and serum levels reflect the levels of iron stored within cells. Serum ferritin is an easily accessible surrogate for stainable bone marrow iron. The iron studies are used collectively to assess the iron status of an individual. Table 19-1 shows that, as expected, serum ferritin and serum iron values are decreased in iron deficiency anemia. Transferrin levels increase when the hepatocytes detect low iron levels, and research shows that this is a transcriptional and posttranslational response to low iron levels.12 The result is a decline in the iron saturation of transferrin that is more dramatic than might be expected simply from the decrease in serum iron level. TABLE 19-1 Results of Iron Studies in Microcytic, Hypochromic Anemias It is important that specimens for iron studies are drawn while fasting and early in the morning when levels are highest. Iron levels show a diurnal variation, with levels dropping throughout the day.13 Iron absorbed from a meal can falsely elevate levels.14 The amount of hemoglobin in reticulocytes can be assessed on some automated hematology analyzers.15 The reticulocyte hemoglobin value is analogous to the MCH, but for reticulocytes. The MCH is the average weight of hemoglobin per cell across the entire RBC population. Some of the RBCs are nearly 120 days old, whereas others are just 1 to 2 days old. If iron deficiency is developing, the MCH does not change until a substantial proportion of the cells are iron deficient, and the diagnosis is effectively delayed for weeks or months after iron deficient erythropoiesis begins. The reticulocyte hemoglobin value is able to assess iron deficient erythropoiesis within days as the first iron deficient cells leave the bone marrow. It is a sensitive indicator of iron deficiency. Even in stage 2 of iron deficiency, before anemia is apparent, the reticulocyte hemoglobin will be low.15
Disorders of Iron and Heme Metabolism
Case Study
Patient Value
Reference Range
WBCs (× 109/L)
8.5
4.5-11
RBCs (× 1012/L)
1.66
4.3-5.9
Hb (g/dL)
3.0
13.9-16.3
Hct (%)
11
39-55
MCV (fL)
66.3
80-100
MCH (pg)
18.1
25.4-34.6
MCHC (g/dL)
27.3
31-37
RDW (%)
20
11.5-14.5
Platelets (× 109/L)
165.0
150-400
WBC differential
Unremarkable
RBC morphology
Marked anisocytosis, marked poikilocytosis, marked hypochromia, marked microcytosis
General Concepts in Anemia
Iron Deficiency Anemia
Etiology
Inadequate Intake
Pathogenesis
Stage 1
Stage 3
Epidemiology
Laboratory Diagnosis
Screening for Iron Deficiency Anemia
Diagnosis of Iron Deficiency
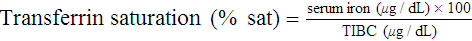
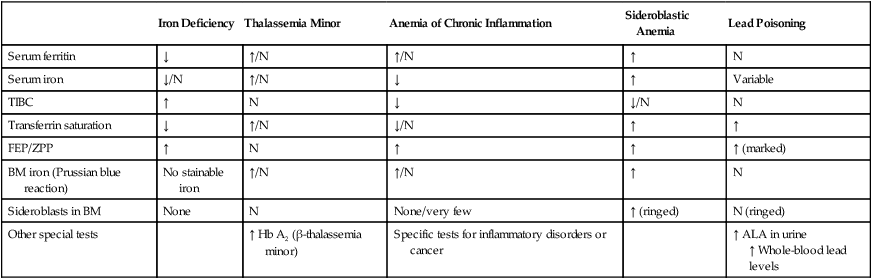
Iron Deficiency
Thalassemia Minor
Anemia of Chronic Inflammation
Sideroblastic Anemia
Lead Poisoning
Serum ferritin
↓
↑/N
↑/N
↑
N
Serum iron
↓/N
↑/N
↓
↑
Variable
TIBC
↑
N
↓
↓/N
N
Transferrin saturation
↓
↑/N
↓/N
↑
↑
FEP/ZPP
↑
N
↑
↑
↑ (marked)
BM iron (Prussian blue reaction)
No stainable iron
↑/N
↑/N
↑
N
Sideroblasts in BM
None
N
None/very few
↑ (ringed)
N (ringed)
Other special tests
↑ Hb A2 (β-thalassemia minor)
Specific tests for inflammatory disorders or cancer
↑ ALA in urine
↑ Whole-blood lead levels
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Disorders of Iron and Heme Metabolism