William A. Rutala, David J. Weber
Disinfection, Sterilization, and Control of Hospital Waste
Each year in the United States there are approximately 53 million outpatient surgical procedures and 46 million inpatient surgical procedures.1 For example, there are at least 10 million gastrointestinal endoscopies per year.2 Each of these procedures involves contact by a medical device or surgical instrument with a patient’s sterile tissue or mucous membranes. A major risk of all such procedures is the introduction of infection. Failure to properly disinfect or sterilize equipment carries not only the risk associated with breach of the host barriers but also the additional risk for person-to-person transmission (e.g., hepatitis B virus) and transmission of environmental pathogens (e.g., Clostridium difficile).
Achieving disinfection and sterilization through the use of disinfectants and sterilization practices is essential for ensuring that medical and surgical instruments do not transmit infectious pathogens to patients. Because it is unnecessary to sterilize all patient care items, health care policies must identify whether cleaning, disinfection, or sterilization is indicated based primarily on the item’s intended use.
Multiple studies in many countries have documented lack of compliance with established guidelines for disinfection and sterilization.3,4 Failure to comply with scientifically based guidelines has led to numerous outbreaks of infectious diseases.2,4–8 In this chapter, which is an update of previous chapters,9–13 a pragmatic approach to the judicious selection and proper use of disinfection and sterilization processes is presented, based on well-designed studies assessing the efficacy (via laboratory investigations) and effectiveness (via clinical studies) of disinfection and sterilization procedures. In addition, we briefly review the management of medical waste in health care facilities.
Definition of Terms
Sterilization is the complete elimination or destruction of all forms of microbial life and is accomplished in health care facilities by either physical or chemical processes. Steam under pressure, dry heat, ethylene oxide (ETO) gas, hydrogen peroxide gas plasma, vaporized hydrogen peroxide, and liquid chemicals are the principal sterilizing agents used in health care facilities. Sterilization is intended to convey an absolute meaning, not a relative one. Unfortunately, some health care professionals as well as the technical and commercial literature refer to “disinfection” as “sterilization” and items as “partially sterile.” When chemicals are used for the purposes of destroying all forms of microbiologic life, including fungal and bacterial spores, they may be called chemical sterilants. These same germicides used for shorter exposure periods may also be part of the disinfection process (i.e., high-level disinfection).
Disinfection describes a process that eliminates many or all pathogenic microorganisms on inanimate objects, with the exception of bacterial spores. Disinfection is usually accomplished by the use of liquid chemicals or wet pasteurization in health care settings. The efficacy of disinfection is affected by a number of factors, each of which may nullify or limit the efficacy of the process. Some of the factors that affect both disinfection and sterilization efficacy are the prior cleaning of the object; the organic and inorganic load present; the type and level of microbial contamination; the concentration of and exposure time to the germicide; the nature of the object (e.g., crevices, hinges, and lumens); the presence of biofilms; the temperature and pH of the disinfection process; and, in some cases, the relative humidity of the sterilization process (e.g., with ETO).
By definition then, disinfection differs from sterilization by its lack of sporicidal property, but this is an oversimplification. A few disinfectants will kill spores with prolonged exposure times (e.g., 3 to 12 hours) and are called chemical sterilants. At similar concentrations but with shorter exposure periods (e.g., 12 minutes for 0.55% ortho-phthalaldehyde) these same disinfectants will kill all microorganisms with the exception of large numbers of bacterial spores and are called high-level disinfectants. Low-level disinfectants may kill most vegetative bacteria, some fungi, and some viruses in a practical period of time (≤10 minutes), whereas intermediate-level disinfectants may be cidal for mycobacteria, vegetative bacteria, most viruses, and most fungi but do not necessarily kill bacterial spores. The germicides differ markedly among themselves primarily in their antimicrobial spectrum and rapidity of action.
Cleaning, on the other hand, is the removal of visible soil (e.g., organic and inorganic material) from objects and surfaces, and it normally is accomplished by manual or mechanical means using water with detergents or enzymatic products. Thorough cleaning is essential before high-level disinfection and sterilization because inorganic and organic materials that remain on the surfaces of instruments interfere with the effectiveness of these processes. Also, if the soiled materials become dried or baked onto the instruments, the removal process becomes more difficult and the disinfection or sterilization process less effective or ineffective. Surgical instruments should be presoaked or rinsed to prevent drying of blood and to soften or remove blood from the instruments. Decontamination is a procedure that removes pathogenic microorganisms from objects so they are safe to handle, use, or discard.
Terms with a suffix “-cide” or “-cidal” for killing action also are commonly used. For example, a germicide is an agent that can kill microorganisms, particularly pathogenic organisms (“germs”). The term germicide includes both antiseptics and disinfectants. Antiseptics are germicides applied to living tissue and skin, whereas disinfectants are antimicrobial agents applied only to inanimate objects. Preservatives are agents that inhibit the growth of microorganisms capable of causing biologic deterioration of substances/materials. In general, antiseptics are only used on the skin and not for surface disinfection and disinfectants are rarely used for skin antisepsis because they may cause injury to skin and other tissues. Other words with the suffix “-cide” (e.g., virucide, fungicide, bactericide, sporicide, and tuberculocide) can kill the type of microorganism identified by the prefix. For example, a bactericide is an agent that kills bacteria.14–19
Rational Approach to Disinfection and Sterilization
About 45 years ago, Earle H. Spaulding15 devised a rational approach to disinfection and sterilization of patient care items or equipment. This classification scheme is so clear and logical that it has been retained, refined, and successfully used by infection control professionals and others when planning methods for disinfection or sterilization.* Spaulding believed that the nature of disinfection could be understood more readily if instruments and items for patient care were divided into three categories based on the degree of risk for infection involved in the use of the items. Although the scheme remains valid, some examples of disinfection studies with viruses, mycobacteria, and protozoa challenge the current definitions and expectations of high- and low-level disinfection.22 The three categories Spaulding described were critical, semicritical, and noncritical.
Critical Items
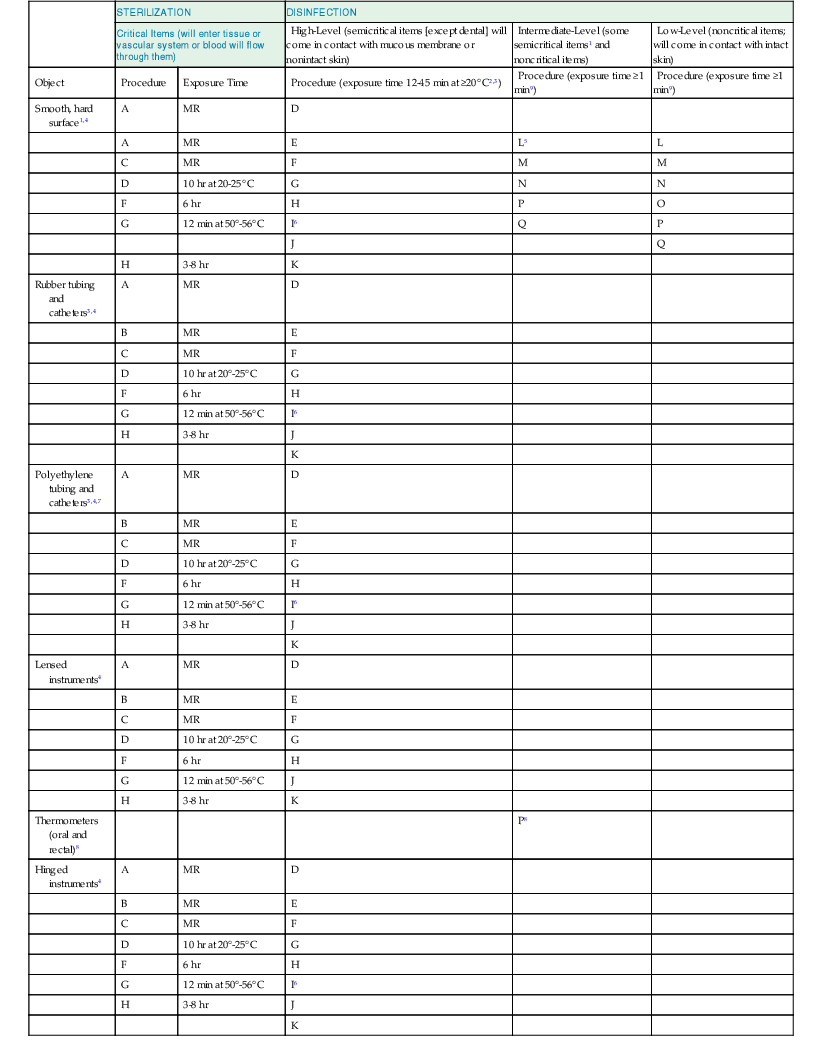
Critical items are so called because of the high risk for infection if such an item is contaminated with any microorganism, including bacterial spores. Thus, it is critical that objects that enter sterile tissue or the vascular system be sterile because any microbial contamination could result in disease transmission. This category includes surgical instruments, cardiac and urinary catheters, implants, arthroscopes, laparoscopes, and ultrasound probes used in sterile body cavities. Most of the items in this category should be purchased in sterile form or be sterilized by steam sterilization if possible. If heat sensitive, the object may be treated with ETO, hydrogen peroxide gas plasma, hydrogen peroxide vapor, or liquid chemical sterilants if other methods are unsuitable. Tables 301-1 and 301-2 list several germicides categorized as chemical sterilants and high-level disinfectants. These include 2.4% or greater glutaraldehyde-based formulations, hypochlorous acid/hypochlorite 650 to 675 ppm free chlorine, 1.12% glutaraldehyde with 1.93% phenol/phenate, 3.4% glutaraldehyde with 26% isopropanol,23 7.5% stabilized hydrogen peroxide, 2.0% hydrogen peroxide, 7.35% hydrogen peroxide with 0.23% peracetic acid, 8.3% hydrogen peroxide with 7.0% peracetic acid, 0.2% peracetic acid, 0.55% or greater ortho-phthalaldehyde, and 0.08% peracetic acid with 1.0% hydrogen peroxide.24 Liquid chemical sterilants can be relied on to produce sterility only if cleaning (to eliminate organic and inorganic material) precedes treatment and if proper use as to concentration, contact time, temperature, and pH is met.25
TABLE 301-1
Methods of Sterilization and Disinfection
| STERILIZATION | DISINFECTION | ||||
| Critical Items (will enter tissue or vascular system or blood will flow through them) | High-Level (semicritical items [except dental] will come in contact with mucous membrane or nonintact skin) | Intermediate-Level (some semicritical items1 and noncritical items) | Low-Level (noncritical items; will come in contact with intact skin) | ||
| Object | Procedure | Exposure Time | Procedure (exposure time 12-45 min at ≥20° C2,3) | Procedure (exposure time ≥1 min9) | Procedure (exposure time ≥1 min9) |
| Smooth, hard surface1,4 | A | MR | D | ||
| A | MR | E | L5 | L | |
| C | MR | F | M | M | |
| D | 10 hr at 20-25° C | G | N | N | |
| F | 6 hr | H | P | O | |
| G | 12 min at 50°-56° C | I6 | Q | P | |
| J | Q | ||||
| H | 3-8 hr | K | |||
| Rubber tubing and catheters3,4 | A | MR | D | ||
| B | MR | E | |||
| C | MR | F | |||
| D | 10 hr at 20°-25° C | G | |||
| F | 6 hr | H | |||
| G | 12 min at 50°-56° C | I6 | |||
| H | 3-8 hr | J | |||
| K | |||||
| Polyethylene tubing and catheters3,4,7 | A | MR | D | ||
| B | MR | E | |||
| C | MR | F | |||
| D | 10 hr at 20°-25° C | G | |||
| F | 6 hr | H | |||
| G | 12 min at 50°-56° C | I6 | |||
| H | 3-8 hr | J | |||
| K | |||||
| Lensed instruments4 | A | MR | D | ||
| B | MR | E | |||
| C | MR | F | |||
| D | 10 hr at 20°-25° C | G | |||
| F | 6 hr | H | |||
| G | 12 min at 50°-56° C | J | |||
| H | 3-8 hr | K | |||
| Thermometers (oral and rectal)8 | P8 | ||||
| Hinged instruments4 | A | MR | D | ||
| B | MR | E | |||
| C | MR | F | |||
| D | 10 hr at 20°-25° C | G | |||
| F | 6 hr | H | |||
| G | 12 min at 50°-56° C | I6 | |||
| H | 3-8 hr | J | |||
| K | |||||
1 See text for discussion of hydrotherapy.
2 The longer the exposure to a disinfectant, the more likely it is that all microorganisms will be eliminated. Twenty-minute exposure at 20° C is the minimum time needed to reliably kill Mycobacterium tuberculosis and nontuberculous mycobacteria with 2% glutaraldehyde. With the exception of >2% glutaraldehyde (see text), follow the FDA-cleared high-level disinfection claim. Some high-level disinfectants have a reduced exposure time (e.g., OPA at 12 minutes at 20° C) because of their rapid activity against mycobacteria or reduced exposure time due to increased mycobactericidal activity at elevated temperature (e.g., 2.5% glutaraldehyde at 5 minutes at 35° C, 0.55% OPA at 5 minutes at 25° C in automated endoscope reprocessor).
3 Tubing must be completely filled for high-level disinfection and liquid chemical sterilization; care must be taken to avoid entrapment of air bubbles during immersion.
4 Material compatibility should be investigated when appropriate.
5 A concentration of 1000 ppm available chlorine should be considered where cultures or concentrated preparations of microorganisms have spilled (5.25% to 6.15% household bleach diluted 1 : 50 provides >1000 ppm available chlorine). This solution may corrode some surfaces.
6 Pasteurization (washer-disinfector) of respiratory therapy or anesthesia equipment is a recognized alternative to high-level disinfection. Some data challenge the efficacy of some pasteurization units.
7 Thermostability should be investigated when appropriate.
8 Do not mix rectal and oral thermometers at any stage of handling or processing.
9 By law, all applicable label instructions on EPA-registered products must be followed. If the user selects exposure conditions that differ from those on the EPA-registered products label, the user assumes liability from any injuries resulting from off-label use and is potentially subject to enforcement action under the Federal Insecticide, Fungicide, and Rodenticide Act.
A. Heat sterilization, including steam or hot air (see manufacturer’s recommendations, steam sterilization processing time from 4 to 30 minutes).
B. Ethylene oxide gas (see manufacturer’s recommendations, generally 2 to 6 hours processing time plus aeration time of 8 to 12 hours at 50° to 60° C).
C. Hydrogen peroxide gas plasma (see manufacturer’s recommendations for internal diameter and length restrictions, processing time between 24 to 47 minutes) and vaporized hydrogen peroxide (see manufacturer’s recommendations for internal diameter and length restrictions).
D. Glutaraldehyde-based formulations: ≥2% glutaraldehyde (caution should be exercised with all glutaraldehyde formulations when further in-use dilution is anticipated); glutaraldehyde (1.12%) with 1.93% phenol/phenate; and glutaraldehyde (3.4%) with isopropanol (26%). One glutaraldehyde-based product has a high-level disinfection claim of 5 minutes at 35° C.
E. Ortho-phthalaldehyde (OPA) 0.55%.
F. Hydrogen peroxide, standard 7.5% (will corrode copper, zinc, and brass).
G. Peracetic acid, concentration variable but ≥0.2% is sporicidal. A 0.2% peracetic acid immersion reprocessor operates at 50° to 56° C. Per guidance from the FDA, most hospitals use the 0.2% peracetic acid reprocessor for reprocessing semicritical items that require high-level disinfection. Thus, as a general rule, the reprocessor will not be used to reprocess critical items because critical items should be sterile and with the reprocessor using 0.2% peracetic acid the final processed device cannot be assured to be sterile. Thus, heat-sensitive critical devices should be sterilized by other validated, FDA-cleared, sterilization processes such as hydrogen peroxide gas plasma, ethylene oxide, and vaporized hydrogen peroxide. If a heat-sensitive critical device truly cannot be processed by any other modality than the reprocessor using 0.2% peracetic acid, then the decision is between not using the device at all or reprocessing it in the 0.2% peracetic acid reprocessor (at 50° to 56° C). The decision to use the 0.2% peracetic acid reprocessor at 50° to 56° C for a heat-sensitive critical item that cannot be processed by an alternative sterilization process should be made on a case-by-case basis.
H. Hydrogen peroxide (7.35%) with 0.23% peracetic acid; hydrogen peroxide 1% with peracetic acid 0.08%; 8.3% hydrogen peroxide with 7.0% peracetic acid (will corrode metal instruments).
I. Wet pasteurization at 70°C for 30 minutes with detergent cleaning.
J. Hypochlorite, single-use chlorine generated on site by electrolyzing saline containing >400 to 675 active free chlorine (will corrode metal instruments).
K. Improved hydrogen peroxide ≥2%.
L. Sodium hypochlorite (5.25% to 6.15% household bleach diluted 1 : 500 provides >100 ppm available chlorine).
M. Phenolic germicidal detergent solution (follow product label for use-dilution).
N. Iodophor germicidal detergent solution (follow product label for use-dilution).
O. Quaternary ammonium germicidal detergent solution (follow product label for use-dilution).
P. Ethyl and isopropyl alcohol 60% to 95%.
Q. Improved hydrogen peroxide 0.5% and 1.4%.
EPA, U.S. Environmental Protection Agency; FDA, U.S. Food and Drug Administration; MR, manufacturer’s recommendations; NA, not applicable.
Note: The selection and use of disinfectants in the health care field is dynamic, and products may become available that are not in existence when this chapter was written. As newer disinfectants become available, persons or committees responsible for selecting disinfectants and sterilization processes should be guided by products cleared by the FDA and the EPA as well as by information in the scientific literature and manufacturer recommendations.
Modified from the works of Rutala and Simmons and their colleagues.9,10,13,16,18,19,303
TABLE 301-2
Summary of Advantages and Disadvantages of Chemical Agents Used as Chemical Sterilants or as High-Level Disinfectants
| STERILANT OR DISINFECTANT | ADVANTAGES | DISADVANTAGES |
| Peracetic acid/hydrogen peroxide | No activation required Irritation not significant | Material compatibility concerns (lead, brass, copper, zinc) both cosmetic and functional Limited clinical experience Potential for eye and skin damage |
| Glutaraldehyde | Numerous use studies published Relatively inexpensive Excellent material compatibility | Respiratory irritation from glutaraldehyde vapor Pungent and irritating odor Relatively slow mycobactericidal activity (unless other disinfectants added such as phenolic, alcohol) Coagulates blood and fixes tissue to surfaces Allergic contact dermatitis |
| Hydrogen peroxide, standard | No activation required May enhance removal of organic matter and organisms No disposal issues No odor or irritation issues Does not coagulate blood or fix tissues to surfaces Inactivates Cryptosporidium at high concentrations (e.g., 7.5%) Use studies published | Material compatibility concerns (brass, zinc, copper, and nickel/silver plating) both cosmetic and functional Serious eye damage with contact Some studies show limited bactericidal activity of standard 3% |
| Ortho-phthalaldehyde | Fast-acting high-level disinfectant No activation required Odor not significant Excellent materials compatibility claimed Efficacy data published Does not coagulate blood or fix tissues to surfaces claimed | Stains protein gray (e.g., skin, mucous membranes, clothing, and environmental surfaces) More expensive than glutaraldehyde Eye irritation with contact Slow sporicidal activity Contraindicated for urologic instruments due to anaphylaxis |
| Peracetic acid | Rapid cycle time (30-45 min) Elevated temperature (50°-55° C) liquid immersion Environmental friendly by-products (acetic acid, O2, H2O) Fully automated endoscope reprocessing system Single-use system eliminates need for concentration testing Standardized cycle May enhance removal of organic material and endotoxin No adverse health effects to operators under normal operating conditions Compatible with many materials and instruments Does not coagulate blood or fix tissues to surfaces Sterilant flows through scope facilitating salt, protein, and microbe removal Rapidly sporicidal Provides procedure standardization (constant dilution, perfusion of channel, temperatures, exposure) | Potential material incompatibility (e.g., aluminum anodized coating becomes dull) Used for immersible instruments only One scope or a small number of instruments can be processed in a cycle More expensive (endoscope repairs, operating costs, purchase costs) than high-level disinfection Serious eye and skin damage (concentrated solution) with contact Point-of-use system, no long-term storage |
| Improved hydrogen peroxide (≥2.0%) | No activation required No odor Nonstaining No special venting requirements Manual or automated applications 12-month shelf life, 14-day reuse 8 min at 20° C high-level disinfectant claim | Material compatibility concerns due to limited clinical experience Organic material resistance concerns due to limited data Limited clinical use and comparative microbicidal efficacy data No measurable activity against Clostridium difficile spores |
Note: All products effective in presence of organic soil, relatively easy to use, and have a broad spectrum of antimicrobial activity (bacteria, fungi, viruses, spores, and mycobacteria). The above characteristics are documented in the literature; contact the manufacturer of the instrument and sterilant for additional information.
Semicritical Items
Semicritical items are those that come in contact with mucous membranes or nonintact skin. Respiratory therapy and anesthesia equipment, some endoscopes, laryngoscope blades and handles,26 esophageal manometry probes, endocavitary probes,26 nasopharyngoscopes, prostate biopsy probes,27 infrared coagulation device,28 anorectal manometry catheters, cystoscopes,29 and diaphragm fitting rings are included in this category.26 These medical devices should be free of all microorganisms, although small numbers of bacterial spores may be present. Intact mucous membranes, such as those of the lungs or the gastrointestinal tract, generally are resistant to infection by common bacterial spores but susceptible to other organisms such as bacteria, mycobacteria, and viruses. Semicritical items minimally require high-level disinfection using chemical disinfectants. Glutaraldehyde, hydrogen peroxide, ortho-phthalaldehyde, peracetic acid, and peracetic acid with hydrogen peroxide are cleared by the U.S. Food and Drug Administration (FDA) and are dependable high-level disinfectants provided the factors influencing germicidal procedures are met (see Tables 301-1 and 301-2). When a disinfectant is selected for use with certain patient care items, the chemical compatibility after extended use with the items to be disinfected also must be considered.
The complete elimination of all microorganisms in or on an instrument, with the exception of small numbers of bacterial spores, is the traditional definition of high-level disinfection. The FDA’s definition of high-level disinfection is a sterilant used for a shorter contact time to achieve at least a 6-log10 kill of an appropriate Mycobacterium species. Cleaning followed by high-level disinfection should eliminate sufficient pathogens to prevent transmission of infection.30,31
Semicritical items should be rinsed with sterile water after high-level disinfection to prevent their contamination with organisms that may be present in tap water, such as nontuberculous mycobacteria,8,32 Legionella,33,34 or gram-negative bacilli such as Pseudomonas.18,20,35–37 In circumstances where rinsing with sterile water rinse is not feasible, a tap water or filtered water (0.2-µm filter) rinse should be followed by an alcohol rinse and forced air drying.9,37–39 Forced-air drying markedly reduces bacterial contamination of stored endoscopes, most likely by removing the wet environment favorable for bacterial growth.38 After rinsing, items should be dried and stored (e.g., packaged) in a manner that protects them from recontamination.
Some items that may come in contact with nonintact skin for a brief period of time (i.e., hydrotherapy tanks, bed side rails) are usually considered noncritical surfaces and are disinfected with low- or intermediate-level disinfectants (i.e., phenolic, iodophor, alcohol, chlorine).40 Because hydrotherapy tanks have been associated with spread of infection, some facilities have chosen to disinfect them with recommended levels of chlorine.40
Noncritical Items
Noncritical items are those that come in contact with intact skin but not mucous membranes. Intact skin acts as an effective barrier to most microorganisms; therefore, the sterility of items that come in contact with intact skin is “not critical.” Examples of noncritical items are bedpans, blood pressure cuffs, crutches, bed rails, bedside tables, patient furniture, and floors. The five most commonly touched noncritical items in the patient environment have been quantitatively shown to be bed rails, bed surface, supply cart, overbed table, and intravenous-line pump.41 In contrast to critical and some semicritical items, most noncritical reusable items may be decontaminated where they are used and do not need to be transported to a central processing area. There is virtually no documented risk of transmitting infectious agents to patients via noncritical items36 when they are used as noncritical items and do not contact nonintact skin or mucous membranes. However, these items (e.g., bedside tables, bed rails) could potentially contribute to secondary transmission by contaminating hands of health care workers or by contact with medical equipment that will subsequently come in contact with patients.14,42–45 Table 301-1 lists several low-level disinfectants that may be used for noncritical items. The exposure time listed in Table 301-1 is equal to or greater than 1 minute. Many U.S. Environmental Protection Agency (EPA)-registered disinfectants have a 10-minute label claim. However, multiple investigators have demonstrated the effectiveness of these disinfectants against vegetative bacteria (e.g., Listeria, Escherichia coli, Salmonella, vancomycin-resistant enterococci [VRE], methicillin-resistant Staphylococcus aureus [MRSA]), yeasts (e.g., Candida), mycobacteria (e.g., Mycobacterium tuberculosis), and viruses (e.g., poliovirus) at exposure times of 30 to 60 seconds.42–58 Thus, it is acceptable to disinfect noncritical medical equipment (e.g., blood pressure cuff) and noncritical surfaces (e.g., bedside table) with an EPA-registered disinfectant or disinfectant/detergent at the proper use-dilution and a contact time of at least 1 minute.9,59 Because the typical drying time for a germicide on a surface is 1 to 3 minutes (unless the product contains alcohol [e.g., a 60% to 70% alcohol will dry in about 30 seconds]) (N. Omidbakhsh, written communication), one application of the germicide on all hand contact surfaces to be disinfected is recommended.
Mops (microfiber and cotton string), reusable cleaning cloths, and disposable wipes are regularly used to achieve low-level disinfection.60,61 Microfiber mops have demonstrated superior microbial removal compared with cotton string mops when used with detergent cleaner (95% vs. 68%, respectively). Use of a disinfectant did significantly improve microbial removal when a cotton string mop was used.61 Mops (especially cotton-string mops) are commonly not kept adequately cleaned and disinfected, and if the water-disinfectant mixture is not changed regularly (e.g., after every 3 to 4 rooms, no longer than 60-minute intervals), the mopping procedure may actually spread heavy microbial contamination throughout the health care facility.62 In one study, standard laundering provided acceptable decontamination of heavily contaminated mop heads but chemical disinfection with a phenolic was less effective.62 The frequent laundering of cotton-string mops (e.g., daily) is, therefore, recommended.
Hospital cleanliness continues to attract patient attention and in the United States it is still primarily assessed via visual appearance, which is not a reliable indicator of surface cleanliness.63 Three other methods have been offered for monitoring patient room hygiene and they include adenosine triphosphate (ATP) bioluminescence,64,65 fluorescent markers,66,67 and microbiologic sampling.65 Studies have demonstrated suboptimal cleaning by aerobic colony counts as well as the use of the ATP bioluminescence and fluorescent markers.64,66 ATP bioluminescence and fluorescent markers are preferred to aerobic plate counts because they provide an immediate assessment of cleaning effectiveness.
Disinfection of Health Care Equipment and Surfaces
A great number of disinfectants are used alone or in combinations (e.g., hydrogen peroxide and peracetic acid) in the health care setting. These include alcohols, chlorine and chlorine compounds, formaldehyde, glutaraldehyde, ortho-phthalaldehyde, standard and improved hydrogen peroxide, iodophors, peracetic acid, phenolics, and quaternary ammonium compounds. With some exceptions (e.g., ethanol or bleach), commercial formulations based on these chemicals are considered unique products and must be registered with the EPA or cleared by the FDA. In most instances, a given product is designed for a specific purpose and is to be used in a certain manner. Therefore, the label should be read carefully to ensure that the right product is selected for the intended use and applied in an efficient manner. Additionally, caution must be exercised to avoid hazards with the use of cleaners and disinfectants on electronic medical equipment. Problems associated with the inappropriate use of liquids on electronic medical equipment have included equipment fires, equipment malfunctions, and health care worker burns.68
Disinfectants are not interchangeable and an overview of the performance characteristics of each is provided in the next section so the user has sufficient information to select an appropriate disinfectant for any item and use it in the most efficient way. It should be recognized that excessive costs may be attributed to incorrect concentrations and inappropriate disinfectants. Finally, occupational diseases among cleaning personnel have been associated with the use of several disinfectants, such as formaldehyde, glutaraldehyde, and chlorine, and precautions (e.g., gloves, proper ventilation) should be used to minimize exposure.69 Asthma and reactive airway disease may occur in sensitized individuals exposed to any airborne chemical, including germicides. Clinically important asthma may occur at levels below ceiling levels regulated by the Occupational and Safety Health Administration (OSHA) or recommended by the National Institute for Occupational Safety and Health. The preferred method of control is to eliminate the chemical (via engineering controls, or substitution) or relocate the worker.
Chemical Disinfectants
Alcohol
In the health care setting, “alcohol” refers to two water-soluble chemical compounds, the germicidal characteristics of which are generally underrated: ethyl alcohol and isopropyl alcohol.70 These alcohols are rapidly bactericidal rather than bacteriostatic against vegetative forms of bacteria; they also are tuberculocidal, fungicidal, and virucidal but do not destroy bacterial spores. Their cidal activity drops sharply when diluted below 50% concentration, and the optimal bactericidal concentration is in the range of 60% to 90% solutions in water (volume/volume).71,72
Alcohols are not recommended for sterilizing medical and surgical materials, principally because of their lack of sporicidal action and their inability to penetrate protein-rich materials. Fatal postoperative wound infections with Clostridium have occurred when alcohols were used to sterilize surgical instruments contaminated with bacterial spores.73 Alcohols have been used effectively to disinfect oral and rectal thermometers, computers,60 hospital pagers, scissors, cardiopulmonary resuscitation (CPR) manikins, applanation tonometers,74 external surfaces of equipment (e.g., ventilators), and stethoscopes.75 Alcohol towelettes have been used for years to disinfect small surfaces such as rubber stoppers of multiple-dose medication vials or vaccine bottles.
Alcohols are flammable and consequently must be stored in a cool, well-ventilated area. They also evaporate rapidly, and this makes extended exposure time difficult to achieve unless the items are immersed.
Chlorine and Chlorine Compounds
Hypochlorites are the most widely used of the chlorine disinfectants and are available in liquid (e.g., sodium hypochlorite) or solid (e.g., calcium hypochlorite) forms. The most prevalent chlorine products in the United States are aqueous solutions of 5.25% to 6.15% sodium hypochlorite, which usually are called household bleach. They have a broad spectrum of antimicrobial activity (i.e., bactericidal, virucidal, fungicidal, mycobactericidal, sporicidal), do not leave toxic residues, are unaffected by water hardness, are inexpensive and fast acting,74,76 remove dried or fixed organisms and biofilms from surfaces,77 and have a low incidence of serious toxicity.78,79 Sodium hypochlorite at the concentration used in domestic bleach (5.25% to 6.15%) may produce ocular irritation or oropharyngeal, esophageal, and gastric burns.69,80,81 Other disadvantages of hypochlorites include corrosiveness to metals in high concentrations (>500 ppm), inactivation by organic matter, discoloring or “bleaching” of fabrics, release of toxic chlorine gas when mixed with ammonia or acid (e.g., household cleaning agents),82 and relative stability.83
Reports have examined the microbicidal activity of a new disinfectant, “superoxidized water.” The concept of electrolyzing saline to create a disinfectant or antiseptic is appealing because the basic materials of saline and electricity are inexpensive and the end product (i.e., water) is not damaging to the environment. The main products of this “water” are hypochlorous acid (e.g., at a concentration of about 144 mg/L) and chlorine. This is also known as electrolyzed water; and, as with any germicide, the antimicrobial activity of superoxidized water is strongly affected by the concentration of the active ingredient (available free chlorine).84 The free available chlorine concentrations of different superoxidized solutions reported in the literature range from 7 to 180 ppm.84 Data have shown that freshly generated superoxidized water is rapidly effective (<2 minutes) in achieving a 5-log10 reduction of pathogenic microorganisms (i.e., M. tuberculosis, Mycobacterium chelonae, poliovirus, human immunodeficiency virus (HIV), MRSA, E. coli, Candida albicans, Enterococcus faecalis, Pseudomonas aeruginosa) in the absence of organic loading. However, the biocidal activity of this disinfectant was substantially reduced in the presence of organic material (5% horse serum).85,86
Hypochlorites are widely used in health care facilities in a variety of settings.76 Inorganic chlorine solution is used to disinfect tonometer heads87 and for disinfection of noncritical surfaces and equipment. A 1 : 10 to 1 : 100 dilution of 5.25% to 6.15% sodium hypochlorite (i.e., household bleach)88–91 or an EPA-registered tuberculocidal disinfectant18 has been recommended for decontaminating blood spills. For small spills of blood (i.e., drops of blood) on noncritical surfaces, the area can be disinfected with a 1 : 100 dilution of 5.25% to 6.15% sodium hypochlorite or an EPA-registered tuberculocidal disinfectant. Because hypochlorites and other germicides are substantially inactivated in the presence of blood,54,92 large spills of blood require that the surface be cleaned before an EPA-registered disinfectant or a 1 : 10 (final concentration) solution of household bleach is applied. If there is a possibility of a sharps injury, there should be an initial decontamination,69,93 followed by cleaning and terminal disinfection (1 : 10 final concentration).54 Extreme care should always be used to prevent percutaneous injury. At least 500 ppm available chlorine for 10 minutes is recommended for decontamination of CPR training manikins. Other uses in health care include as an irrigating agent in endodontic treatment and to disinfect laundry, dental appliances, hydrotherapy tanks,40 regulated medical waste before disposal,76 applanation tonometers,74 and the water distribution system in hemodialysis centers and hemodialysis machines.9,75 Disinfection with a 1 : 10 dilution of concentrated sodium hypochlorite (i.e., bleach) has been shown to be effective in reducing environmental contamination in patient rooms and in reducing C. difficile infection rates in hospital units where there is a high endemic C. difficile infection rates or in an outbreak setting.9,94–96,97,98 At our institution, we use a sporicidal solution (5000 ppm chlorine) in all C. difficile–infected patient rooms for routine daily and terminal cleaning. This is done by one application of the sporicide covering all hand contact surfaces to allow sufficient wetness for a greater than 1-minute contact time.
Chlorine has long been favored as the preferred disinfectant in water treatment. Hyperchlorination of a Legionella-contaminated hospital water system40 resulted in a dramatic decrease (30% to 1.5%) in the isolation of Legionella pneumophila from water outlets and a cessation of health care–associated legionnaires’ disease in the affected unit.99,100 Chloramine T and hypochlorites have been used in disinfecting hydrotherapy equipment.75
Hypochlorite solutions in tap water at a pH greater than 8 stored at room temperature (23° C) in closed, opaque plastic containers may lose up to 40% to 50% of their free available chlorine level over a period of 1 month. Thus, if a user wished to have a solution containing 500 ppm of available chlorine at day 30, a solution containing 1000 ppm of chlorine should be prepared at time 0. There is no decomposition of sodium hypochlorite solution after 30 days when stored in a closed brown bottle.83
Glutaraldehyde
Glutaraldehyde is a saturated dialdehyde that has gained wide acceptance as a high-level disinfectant and chemical sterilant.101 Aqueous solutions of glutaraldehyde are acidic and generally in this state are not sporicidal. Only when the solution is “activated” (made alkaline) by use of alkalinizing agents to pH 7.5 to 8.5 does the solution become sporicidal. Once “activated” these solutions have a shelf life of minimally 14 days because of the polymerization of the glutaraldehyde molecules at alkaline pH levels. This polymerization blocks the active sites (aldehyde groups) of the glutaraldehyde molecules that are responsible for its biocidal activity.
Novel glutaraldehyde formulations (e.g., glutaraldehyde-phenol-sodium phenate, potentiated acid glutaraldehyde, stabilized alkaline glutaraldehyde) produced in the past 40 years have overcome the problem of rapid loss of activity (e.g., now use life of 28 to 30 days) while generally maintaining excellent microbicidal activity.74,75,102,103 However, it should be recognized that antimicrobial activity is dependent not only on age but also on use conditions such as dilution and organic stress. The use of glutaraldehyde-based solutions in health care facilities is common because of their advantages, which include excellent biocidal properties; activity in the presence of organic matter (20% bovine serum); and noncorrosive action to endoscopic equipment, thermometers, rubber, or plastic equipment. The advantages, disadvantages, and characteristics of glutaraldehyde are listed in Table 301-2.
The in vitro inactivation of microorganisms by glutaraldehydes has been extensively investigated and reviewed.104 Several investigators showed that 2% or greater aqueous solutions of glutaraldehyde, buffered to pH 7.5 to 8.5 with sodium bicarbonate, were effective in killing vegetative bacteria in less than 2 minutes; M. tuberculosis, fungi, and viruses in less than 10 minutes; and spores of Bacillus and Clostridium species in 3 hours.104 Spores of C. difficile are more rapidly killed by 2% glutaraldehyde than are spores of other species of Clostridium and Bacillus,105,106 and this includes the hypervirulent binary toxin strains of C. difficile spores (W.A. Rutala, unpublished data, December 2012). There have been reports of microorganisms with relative resistance to glutaraldehyde, including some mycobacteria (M. chelonae, Mycobacterium avium-intracellulare, Mycobacterium xenopi),107–109 Methylobacterium mesophilicum,110 Trichosporon, fungal ascospores (e.g., Microascus cinereus, Chaetomium globosum), and Cryptosporidium.111 M. chelonae persisted in a 0.2% glutaraldehyde solution used to store porcine prosthetic heart valves,112 and a large outbreak of Mycobacterium massiliense infections in Brazil after videolaparoscopy equipment used for different elective cosmetic procedures (e.g., liposuction) was highly tolerant to 2% glutaraldehyde.113 Porins may have a role in the resistance of mycobacteria to glutaraldehyde and ortho-phthalaldehyde.114
Dilution of glutaraldehyde during use commonly occurs, and studies show a glutaraldehyde concentration decline after a few days of use in an automatic endoscope washer.115 This decline occurs because instruments are not thoroughly dried and water is carried in with the instrument, which increases the solution’s volume and dilutes its effective concentration. This emphasizes the need to ensure that semicritical equipment is disinfected with an acceptable concentration of glutaraldehyde. Data suggest that 1.0% to 1.5% glutaraldehyde is the minimal effective concentration (MEC) for 2% or greater glutaraldehyde solutions when used as a high-level disinfectant.115–117 Chemical test strips or liquid chemical monitors are available for determining whether an effective concentration of glutaraldehyde is present despite repeated use and dilution. The frequency of testing should be based on how frequently the solutions are used (e.g., used daily, test daily; used weekly, test before use), but the strips should not be used to extend the use life beyond the expiration date. Data suggest the chemicals in the test strip deteriorate with time,118 and a manufacturer’s expiration date should be placed on the bottles. The bottle of test strips should be dated when opened and used for the period of time indicated on the bottle (e.g., 120 days). The results of test strip monitoring should be documented. The glutaraldehyde test kits have been preliminarily evaluated for accuracy and range,118 but their reliability has been questioned.119 The concentration should be considered unacceptable or unsafe when the test indicates a dilution below the product’s MEC (generally to 1.0% to 1.5% glutaraldehyde or lower) by the indicator not changing color.
Glutaraldehyde is used most commonly as a high-level disinfectant for medical equipment such as endoscopes,93 endocavitary probes, spirometry tubing, dialyzers, transducers, anesthesia and respiratory therapy equipment, hemodialysis proportioning and dialysate delivery systems, and reuse of laparoscopic disposable plastic trocars.75 Glutaraldehyde is noncorrosive to metal and does not damage lensed instruments, rubber, or plastics. The FDA-cleared labels for high-level disinfection with 2% or greater glutaraldehyde at 25° C range from 20 to 90 minutes depending on the product. However, multiple scientific studies and professional organizations support the efficacy of 2% or greater glutaraldehyde for 20 minutes at 20° C.9,18,37 Minimally, this latter recommendation should be followed. Glutaraldehyde should not be used for cleaning noncritical surfaces because it is too toxic and expensive.
Colitis believed to be due to glutaraldehyde exposure from residual disinfecting solution in the endoscope solution channels has been reported and is preventable by careful endoscope rinsing.69 One study found that residual glutaraldehyde levels were higher and more variable after manual disinfection (<0.2 to 159.5 mg/L) than after automatic disinfection (0.2 to 6.3 mg/L).120 Similarly, keratopathy and corneal damage were caused by ophthalmic instruments that were inadequately rinsed after soaking in 2% glutaraldehyde.121
Glutaraldehyde exposure should be monitored to ensure a safe work environment. In the absence of an OSHA permissible exposure limit, if the glutaraldehyde level is higher than the American Conference of Industrial Hygienists ceiling limit of 0.05 ppm, it would be prudent to take corrective action and repeat monitoring.122
Hydrogen Peroxide
The literature contains several accounts of the properties, germicidal effectiveness, and potential uses for stabilized hydrogen peroxide in the health care setting. Published reports ascribe good germicidal activity to hydrogen peroxide and attest to its bactericidal, virucidal, sporicidal, and fungicidal properties.123–127 Some other studies have shown limited bactericidal and virucidal activity of standard 3% hydrogen peroxide.58,74 The advantages, disadvantages, and characteristics of hydrogen peroxide are listed in Table 301-2. As with other chemical sterilants, dilution of the hydrogen peroxide must be monitored by regularly testing the MEC (i.e., 7.5 to 6.0%). Compatibility testing by Olympus America of the 7.5% hydrogen peroxide found both cosmetic changes (e.g., discoloration of black anodized metal finishes)93 and functional changes with the tested endoscopes (Olympus, October 15, 1999, written communication).
Commercially available 3% hydrogen peroxide is a stable and effective disinfectant when used on inanimate surfaces. It has been used in concentrations from 3% to 6% for the disinfection of soft contact lenses (e.g., 3% for 2 to 3 hours),123,128 tonometer biprisms, ventilators, fabrics,129 and endoscopes.130 Hydrogen peroxide was effective in spot-disinfecting fabrics in patients’ rooms.129 Corneal damage from a hydrogen peroxide–soaked tonometer tip that was not properly rinsed has been reported.131
Improved Hydrogen Peroxide
An improved hydrogen peroxide–based technology has been introduced into health care for disinfection of noncritical environmental surfaces and patient equipment132 and high-level disinfection of semicritical equipment such as endoscopes.133–135 Improved hydrogen peroxide contains very low levels of anionic or nonionic surfactants or both in an acidic product that act with hydrogen peroxide to produce microbicidal activity. This combination of ingredients speeds the antimicrobial activity of hydrogen peroxide and cleaning efficiency.134,135 Improved hydrogen peroxide is considered safe for humans and equipment and benign for the environment. In fact, improved hydrogen peroxide has the lowest EPA toxicity category (i.e., category IV) based on its oral, inhalation, and dermal toxicity, which means it is practically nontoxic and not an irritant.132,134,136 It is prepared and marketed by several companies in various concentrations (e.g., 0.5% to 7%), and different products may use different terminology for these products, such as “accelerated” or “activated.” Lower concentrations (i.e., 0.5%,1.4%) are designed for the low-level disinfection of noncritical environmental surfaces and patient care objects, whereas the higher concentrations can be used as high-level disinfectants for semicritical medical devices (e.g., endoscopes).
A recent study compared the bactericidal activity of a quaternary ammonium compound with two new improved hydrogen peroxide products. The improved hydrogen peroxide products were superior or similar to the quaternary ammonium compound tested. When the two improved hydrogen peroxide products were compared with standard 0.5%, 1.4%, and 3% hydrogen peroxide formulations, the improved hydrogen peroxide–based environmental surface disinfectants proved to be more effective (>6-log10 reduction) and fast-acting (30-60 seconds) microbicides in the presence of a soil load (to simulate the presence of body fluids) than commercially available hydrogen peroxide. Only 30- to 60-second contact time was studied because longer contact times (e.g., 10 minutes) are not achievable in clinical practice. Additionally, the improved hydrogen peroxide products have an EPA-registered contact time that is substantially less (e.g., 30 seconds, 1 minute for bacteria) than most EPA-registered low-level disinfectants.58 We have also recently shown that the 1.4% activated hydrogen peroxide is very effective in reducing microbial contamination of hospital privacy curtains. In our study, the activated hydrogen peroxide completely eliminated contamination with MRSA and VRE and resulted in a 98.5% reduction in microbes (only Bacillus spp. recoverable). Thus, at our institution, privacy curtains are being disinfected at the grab area by spraying the grab area of the curtain three times with activated hydrogen peroxide at discharge cleaning.
Iodophors
Iodine solutions or tinctures have long been used by health care professionals, primarily as antiseptics on skin or tissue. The FDA has not cleared any liquid chemical sterilant/high level disinfectants with iodophors as the main active ingredient. However, iodophors have been used both as antiseptics and disinfectants. An iodophor is a combination of iodine and a solubilizing agent or carrier; the resulting complex provides a sustained-release reservoir of iodine and releases small amounts of free iodine in aqueous solution. The best known and most widely used iodophor is povidone-iodine, a compound of polyvinylpyrrolidone with iodine. This product and other iodophors retain the germicidal efficacy of iodine but, unlike iodine, are generally nonstaining and are relatively free of toxicity and irritancy.137
There are several reports that documented intrinsic microbial contamination of antiseptic formulations of povidone-iodine and poloxamer-iodine.138–140 It was found that “free” iodine (I2) contributes to the bactericidal activity of iodophors and dilutions of iodophors demonstrate more rapid bactericidal action than does a full-strength povidone-iodine solution. Therefore, iodophors must be diluted according to the manufacturers’ directions to achieve antimicrobial activity.
Published reports on the in vitro antimicrobial efficacy of iodophors demonstrate that iodophors are bactericidal, mycobactericidal, and virucidal but may require prolonged contact times to kill certain fungi and bacterial spores.15,141–144
Besides their use as an antiseptic, iodophors have been used for the disinfection of blood culture bottles and medical equipment such as hydrotherapy tanks and thermometers. Antiseptic iodophors are not suitable for use as hard-surface disinfectants because of concentration differences. Iodophors formulated as antiseptics contain less free iodine than those formulated as disinfectants.145 Iodine or iodine-based antiseptics should not be used on silicone catheters because the silicone tubing may be adversely affected.146
Ortho-phthalaldehyde
Ortho-phthalaldehyde (OPA) is a high-level disinfectant that received FDA clearance in October 1999. It contains at least 0.55% 1,2-benzenedicarboxaldehyde or OPA, and it has supplanted glutaraldehyde as the most commonly used “aldehyde” for high-level disinfection in the United States. OPA solution is a clear, pale-blue liquid with a pH of 7.5. The advantages, disadvantages, and characteristics of OPA are listed in Table 301-2.
Studies have demonstrated excellent microbicidal activity in in vitro studies,74,75,93,111,147–152 including superior mycobactericidal activity (5-log10 reduction in 5 minutes) compared with glutaraldehyde. Walsh and colleagues also found OPA effective (>5-log10 reduction) against a wide range of microorganisms, including glutaraldehyde-resistant mycobacteria and Bacillus atrophaeus spores.150
OPA has several potential advantages compared with glutaraldehyde. It has excellent stability over a wide pH range (pH 3 to 9), is not a known irritant to the eyes and nasal passages, does not require exposure monitoring, has a barely perceptible odor, and requires no activation. OPA, like glutaraldehyde, has excellent material compatibility. A potential disadvantage of OPA is that it stains proteins gray (including unprotected skin) and thus must be handled with caution.93 However, skin staining would indicate improper handling that requires additional training and/or personal protective equipment (gloves, eye and mouth protection, fluid-resistant gowns). OPA residues remaining on inadequately water-rinsed transesophageal echocardiographic probes may leave stains on the patient’s mouth. Meticulous cleaning, use of the correct OPA exposure time (e.g., 12 minutes), and copious rinsing of the probe with water should eliminate this problem. Because OPA has been associated with several episodes of anaphylaxis after cystoscopy,153 the manufacturer has modified its instructions for use of OPA and contraindicates the use of OPA as a disinfectant for reprocessing all urologic instrumentation for patients with a history of bladder cancer. Personal protective equipment should be worn when handling contaminated instruments, equipment, and chemicals.148 In addition, equipment must be thoroughly rinsed to prevent discoloration of a patient’s skin or mucous membrane. The MEC of OPA is 0.3%, and that concentration is monitored by test strips designed specifically for the OPA solution. OPA exposure level monitoring found that the concentration during the disinfection process was significantly higher in the manual group (median, 1.43 ppb) than in the automatic group (median, 0.35 ppb). These findings corroborate other findings that show it is desirable to introduce automatic endoscope reprocessors to decrease disinfectant exposure levels among scope reprocessing technicians.154
Peracetic Acid
Peracetic, or peroxyacetic acid, is characterized by a very rapid action against all microorganisms. A special advantage of peracetic acid is its lack of harmful decomposition products (i.e., acetic acid, water, oxygen, hydrogen peroxide); it enhances removal of organic material155 and leaves no residue. It remains effective in the presence of organic matter and is sporicidal even at low temperatures. Peracetic acid can corrode copper, brass, bronze, plain steel, and galvanized iron, but these effects can be reduced by additives and pH modifications. The advantages, disadvantages, and characteristics of peracetic acid are listed in Table 301-2.
Peracetic acid will inactivate gram-positive and gram-negative bacteria, fungi, and yeasts in less than 5 minutes at less than 100 ppm. In the presence of organic matter, 200 to 500 ppm is required. For viruses the dosage range is wide (12 to 2250 ppm), with poliovirus inactivated in yeast extract in 15 minutes with 1500 to 2250 ppm. A processing system using peracetic acid at a temperature of 50° C to 56° C can be used for processing heat-sensitive semicritical and critical devices that are compatible with the peracetic acid and processing system and cannot be sterilized by other legally marketed traditional sterilization methods validated for that type of device (e.g., steam, hydrogen peroxide gas plasma, vaporized hydrogen peroxide). After processing, the devices should be used immediately or stored in a manner similar to that of a high-level disinfected endoscope.156–158 The sterilant, 35% peracetic acid, is diluted to 0.2% with tap water that has been filtered and exposed to ultraviolet light. Simulated-use trials with the earlier version of this processing system have demonstrated excellent microbicidal activity,74,158–161,162 and three clinical trials have demonstrated both excellent microbial killing and no clinical failures leading to infection.163–165 Three clusters of infection using the earlier version of the peracetic acid automated endoscope reprocessor were linked to inadequately processed bronchoscopes when inappropriate channel connectors were used with the system.166,167 These clusters highlight the importance of training, proper model-specific endoscope connector systems, and quality control procedures to ensure compliance with endoscope manufacturer’s recommendations and professional organization guidelines. An alternative high-level disinfectant available in the United Kingdom contains 0.35% peracetic acid. Although this product is rapidly effective against a broad range of microorganisms,168,169 it tarnishes the metal of endoscopes and is unstable, resulting in only a 24-hour use life.169
Peracetic Acid with Hydrogen Peroxide
Three chemical sterilants are FDA-cleared that contain peracetic acid plus hydrogen peroxide (0.08% peracetic acid plus 1.0% hydrogen peroxide, 0.23% peracetic acid plus 7.35% hydrogen peroxide, and 8.3% hydrogen peroxide plus 7.0% peracetic acid). The advantages, disadvantages, and characteristics of peracetic acid with hydrogen peroxide are listed in Table 301-2.
The bactericidal properties of peracetic acid plus hydrogen peroxide have been demonstrated.170 Manufacturer’s data demonstrated that this combination of peracetic acid plus hydrogen peroxide inactivated all microorganisms with the exception of bacterial spores within 20 minutes. The 0.08% peracetic acid plus 1.0% hydrogen peroxide product was effective in inactivating a glutaraldehyde-resistant mycobacteria.171
The combination of peracetic acid and hydrogen peroxide has been used for disinfecting hemodialyzers.172 The percentage of dialysis centers using a peracetic acid with hydrogen peroxide–based disinfectant for reprocessing dialyzers increased from 5% in 1983 to 72% in 1997.173
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree