Chapter 77 Diseases of the Esophagus, Stomach, and Bowel
The early years of the acquired immunodeficiency syndrome (AIDS) epidemic highlighted the gastrointestinal (GI) tract as a target for a variety of opportunistic disorders. Because of the prevalence of gastrointestinal diseases in these patients, the spectrum of potential etiologies, approach to evaluation and therapy, and indications for prophylaxis have become well established. Although our therapeutic armamentarium has continued to expand, truly effective therapy for some opportunistic infections remains elusive. Fortunately, since the introduction of protease inhibitors (PIs) and highly active antiretroviral therapy (HAART), there has been a major decline of gastrointestinal opportunistic disorders in AIDS patients.1,2 Nevertheless, for areas of the world with limited access to HAART, GI disease remains widely prevalent mirroring the early years of the epidemic in developed countries.3 AIDS-related complications also remain prevalent for those who are noncompliant and recently infected patients.4,5 The long-term prognosis for most GI disorders is dictated primarily by the degree of underlying immunodeficiency.
INFECTIONS OF THE ESOPHAGUS
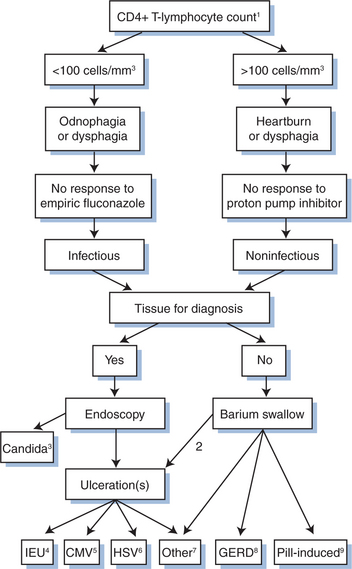
In the pre-HAART era, esophageal infections were common in patients with AIDS with at least one-third of these patients experiencing esophageal symptoms at some point during their illness.6 With HAART, these infections have been reduced in frequency especially those caused by Candida.1,7 When evaluating a patient with esophageal complaints, a clear distinction must be drawn between dysphagia (sensation of food or pills “sticking” in the chest) and odynophagia (substernal pain after swallowing). Other esophageal signs and symptoms resulting from esophageal infections include esophagospasm (spontaneous substernal chest pain), singultus (“hiccups”), and hematemesis. In patients with esophageal infections the physical examination is often unrevealing, except for the presence of thrush, which suggests the presence of esophageal candidiasis. The absence of thrush, however, does not exclude Candida esophagitis.8 Oropharyngeal candidiasis is readily diagnosed by the characteristic multiple white-yellow plaques, which can be focal or completely coat the buccal mucosa. Occasionally, candidiasis manifests as mucosal erythema in the absence of recognizable plaques or angular cheilitis. It is important to differentiate oral hairy leukoplakia (nonremovable white plaques on the lateral aspect of the tongue) from thrush given the different etiologies and therapy.9 Another important fact is that the esophagus may be involved by multiple concurrent processes.10 The main diagnostic tools employed to diagnose esophageal infections in HIV-infected patients are empiric therapy, barium esophagography, and endoscopy. Barium studies are often nonspecific. Endoscopy with biopsy is the gold standard for diagnosis, and in addition, upper endoscopy permits direct visualization of lesions and retrieval of tissue for analysis (Fig. 77-1).
Etiology
The most common infectious cause of esophagitis in patients with HIV infection is Candida. While Candida albicans is by far the most common cause of candidiasis, several other non-albicans species, including C. krusei, C. tropicalis, C. parapsilosis, C. glabrata, and C. dublinensis, have been associated with oral and esophageal candidiasis in HIV-infected individuals, particularly after prolonged antifungal drug therapy.11 Generally, determining the specific Candida species is not required; however, certain species are more often azole resistant than others. For example, the identification of C. krusei suggests that azole therapy is unlikely to be successful. In general, in vitro azole resistance in patients with AIDS correlates with azole treatment failure for oropharyngeal candidiasis.12 The major risk factor associated with the development of resistance was the prophylactic daily or intermittent use of fluconazole within the last 6 months. Fortunately, refractory mucosal candidiasis is now less common during the era of HAART.
Cytomegalovirus (CMV) is one of the most common opportunistic infections in patients with AIDS and typically occurs when the CD4+ T-lymphocyte count is less than 100 cells per cubic millimeter. In such patients who do not receive antiretroviral therapy, the incidence of disease may approach 21% at 2 years.13,14 Although the retina is the most common target for CMV, CMV esophagitis and colitis remain significant causes of morbidity. Involvement of the stomach and small bowel is less common.
Herpes simplex virus (HSV) occasionally causes enteral disease. Because HSV infects primarily squamous mucosa, oropharyngeal, esophageal, and perianal involvement are the most common sites of disease. Oropharyngeal disease may be isolated or can occur in association with esophageal disease. In a large prospective study of 100 HIV-infected patients with ulcerative esophagitis, HSV esophagitis was identified in only 5%, compared to 50% for CMV.15 Like CMV, the incidence of HSV disease rises as immunodeficiency worsens, with the greatest frequency occurring when the CD4+ T-lymphocyte count is less than 100 cells per cubic millimeter.16
An important cause of esophageal ulceration not clearly linked to a specific infection is HIV-associated idiopathic esophageal ulceration (IEU). These lesions can present at the time of seroconversion, although they typically occur when immunodeficiency is severe.15 The median CD4+ T-lymphocyte count in these patients is less than 50 cells per cubic millimeter. Several studies have identified HIV-infected inflammatory cells in the ulcer base of these lesions, suggesting an etiologic role for HIV. However, HIV has not been identified in esophageal squamous mucosa but, rather, in inflammatory cells and has been found in HIV-infected patients with esophageal diseases other than IEU.17 IEUs are almost as common as CMV esophagitis in patients with AIDS, comprising ∼40% of esophageal ulcers in these patients.15
A variety of other infectious agents have been reported to involve the esophagus but are rare; they include protozoa (Cryptosporidium, Pneumocystis carinii, Leishmania donovani, Trichomonas vaginalis), bacteria (Nocardia, Actinomyces), mycobacteria (Mycobacterium avium complex, M. tuberculosis), fungi (Histoplasma capsulatum, Penicillium chrysogenum, Exophiala jeanselmani), and viruses (Epstein–Barr, human papillomavirus)18 (Table 77-1). The numerous causes of esophageal disease in these patients underscore the importance of an accurate diagnosis.
Table 77-1 Etiology of Esophagitis in AIDS Patients
| Fungi | Candida species (Penicillium chrysogenum, Pneumocystis carinii, Exophiala jeanselmani, Cryptococcus neoformans, mucormycosis, Aspergillus fumigatus) |
| Viruses | Cytomegalovirus (CMV), herpes simplex virus type II (Epstein–Barr virus, papovavirus, human herpesvirus (HHV-6)) |
| Idiopathic | idiopathic esophageal ulcer |
| Pill-induced | zalcitabine, zidovudine |
| Peptic | gastroesophageal disease |
| Bacteria | Mycobacterium avium complex (Mycobacterium tuberculosis, Bartonella henselae, Nocardia asteroides, Actinomyces israelii) |
| Protozoa | (Cryptosporidium parvum, Leishmania donovani, Trichomonas vaginalis) |
| Tumors | Non-Hodgkin lymphoma, Kaposi sarcoma (squamous cell carcinoma, adenocarcinoma, lymphomatoid granulomatosis) |
Rare entities are shown in brackets.
Clinical Presentation
Patients with CMV esophagitis usually present with odynophagia which is characteristically severe.15 In contrast to Candida esophagitis, dysphagia is distinctly uncommon. Heartburn is uncommon; nausea, vomiting, and low-grade fever may be reported. Concurrent oropharyngeal ulcerations are rare, whereas thrush is often present.
Esophagitis due to HSV presents similarly to CMV esophagitis. The most common manifestations of esophageal HSV are odynophagia and dysphagia (82%), chest pain (68%), and fever (44%).16 GI bleeding is rare. Ulcers of the oral mucosa, lips, and nares are often but not invariably present.
Diagnosis
Esophageal candidiasis is suspected clinically in the patient with moderately severe immunodeficiency (CD4+ T-lymphocyte count less than 100 cells per cubic millimeter) and esophageal symptoms, with or without thrush. Thrush is absent in one-third of patients with esophageal candidiasis.8 Barium esophagography is relatively insensitive and nonspecific for detecting mild esophagitis and should not be performed for diagnostic purposes. The most common radiographic finding of Candida esophagitis is diffuse mucosal irregularity resulting in a “shaggy” appearance mimicking diffuse ulceration. Endoscopy is the most sensitive diagnostic method. Multiple yellow-white plaques with the appearance of “cottage cheese” are pathognomonic for esophageal candidiasis. A definitive diagnosis rests on the identification of typical yeast forms in endoscopic mucosal biopsies, esophageal brushings, or balloon cytology. The detection of Candida by these methods does not exclude other disorders, as Candida may coexist with additional esophageal processes in up to 25% of symptomatic patients.6,10 Cultures of the esophagus may provide specimens for drug susceptibility testing but because Candida species can be cultured from patients without esophagitis; cultures are not as useful clinically.
On the barium esophagogram CMV may appear as one or multiple well circumscribed ulcers that may be shallow or deep. Endoscopically CMV results in large shallow or deep ulcerations that may be circumferential and may be multiple.19 The diagnosis of CMV disease is best established by identifying a viral cytopathic effect (intranuclear inclusions) in GI mucosal biopsy specimens using routine hematoxylin and eosin (H & E) staining. The cytopathic effect of CMV is present in endothelial cells and mesenchymal cells; thus, it is imperative to obtain biopsy specimens from the ulcer base. Immunohistochemical stains of mucosal biopsies may be required to confirm the infection; viral culture of biopsy specimens is less sensitive and specific.20 Blood cultures, serologic tests for CMV, as well as the presence of CMV antigenemia or viremia, are not useful for diagnosing CMV GI infection in HIV-infected patients, but have been linked to end-organ disease and higher mortality.21,22
IEU appears endoscopically as one or multiple ulcers of variable depth with normal intervening mucosa. CMV esophagitis and IEU are indistinguishable clinically, radiographically, and endoscopically.18 The diagnosis of IEU is one of exclusion; multiple biopsies (at least six) of the ulcer margins and ulcer base are necessary to exclude an infectious process.
Therapy
Given that esophageal candidiasis is the most common cause of esophageal infection in HIV-infected patients, it is common practice to administer an empirical trial of antifungal therapy in patients with esophageal complaints and thrush. A prospective study comparing empirical fluconazole to endoscopy for presumptive esophageal candidiasis in patients with AIDS showed that empirical fluconazole was a safe, cost-effective alternative to immediate endoscopy.23 Fluconazole is administered with an oral or intravenous loading dose of 200 mg followed by 100 mg/day for 10–14 days. Failure to respond clinically in 3–7 days should prompt endoscopy to establish the etiology.
Although topical nystatin is effective for oropharyngeal candidiasis, clotrimazole troches have now largely replaced nystatin as the principal nonsystemic agent, given the ease of administration, palatability, negligible side effects, drug interactions, and effectiveness. Clinical cure is seen in 65–94% of patients following 14 days of clotrimazole therapy.24 The use of systemic therapy with oral fluconazole for oropharyngeal candidiasis in HIV-infected patients achieves greater cure rates and lower relapse rates than clotrimazole troches25 or nystatin.26 Thus, neither nystatin nor clotrimazole is recommended. Single-dose therapy with fluconazole 150 mg is as efficacious as a 7-day treatment course for oral candidiasis.27 Continuous and intermittent therapy are equivalent with refractory disease developing in ∼4% at 42 months.28 Unlike oropharyngeal disease, nonsystemic therapy (e.g., nystatin) is largely ineffective for esophageal candidiasis.
Three orally administered systemic azole antifungal agents have been investigated and used extensively to treat esophageal candidiasis: ketoconazole, fluconazole, and itraconazole. At most centers fluconazole has become the drug of choice for esophageal candidiasis associated with AIDS. Randomized trials have shown fluconazole to have efficacy superior to that of ketoconazole for both oropharyngeal and esophageal candidiasis.25,29 The response rate of esophageal candidiasis to fluconazole tends to be rapid, with most patients experiencing significant clinical improvement within 5 days.23,30 In the largest study reported to date, Barbaro et al31 randomized 2213 HIV-infected patients with a first episode of Candida esophagitis to either fluconazole or itraconazole. Clinical cure was achieved in 81% of fluconazole-treated patients compared to 75% of itraconazole-treated patients (P < 0.001), although there was no difference in clinical and endoscopic cure at the end of the follow-up period (1 year). Approximately 25% of patients in both groups required an increase in dosage at 2 weeks.
Oral suspensions of both fluconazole and itraconazole have been developed, and their efficacy appears similar to that of pills. Results of comparative trials between fluconazole tablets and itraconazole suspension suggest equivalency in clinical response, mycologic eradication, and tolerability.32
Intravenous amphotericin B is highly effective against most Candida species. Because of its toxicity, this drug is used almost exclusively for candidiasis resistant to azole therapy. Lozenges containing amphotericin B and oral suspensions of amphotericin B have some efficacy and are available in Europe but not in the United States.33 Low-dose amphotericin B (0.3–0.5 mg kg−1 day−1 for 7–14 days) is usually adequate therapy for oropharyngeal and esophageal candidiasis. Liposomal forms of amphotericin B are also effective and can be used if cost is not a factor and amphotericin B cannot be tolerated. Improved antiretroviral therapy, possibly by increasing the CD4+ T-lymphocyte count and thereby immune function, also leads to clearance of refractory thrush in some patients.
The newest agents available for candidal infection are the echinocandins.34 These novel agents inhibit fungal cell wall synthesis through the inhibition of glucan synthase. Caspofungin, the first available agent in this class, has activity against both aspergillus and candida species including non-albicans strains. Prospective randomized trials have documented an efficacy and side effect profile similar to fluconazole for Candida esophagitis in AIDS,35 and equal efficacy, better tolerability and comparable relapse rate than amphotericin B for azole resistant esophageal candidiasis.36–38 Therefore, this drug has become the treatment of choice for resistant Candida. Other members of the echinocandin class have been less studied but are similarly effective.39
The incidence of azole-resistant mucosal candidiasis appears to be decreasing largely as a result of HAART. The risk factors, natural history, and outcome of fluconazole-resistant mucosal candidiasis have been examined.40 The major risk factor associated with the development of resistance was the prophylactic daily or intermittent use of fluconazole within the last 6 months. The researchers also found that the prognosis of these patients was worse than that of patients with nonresistant Candida. In patients with fluconazole-resistant mucocutaneous candidiasis, treatment options include itraconazole (oral suspension or intravenous preparations), amphotericin, or more recently caspofungin.
Despite the frequency of oropharyngeal and esophageal candidiasis in HIV-infected patients, primary prophylaxis is not recommended. Although fluconazole prophylaxis decreases the occurrence of disease,41 it is not recommended because these disorders are not life-threatening, acute therapy of individual episodes is highly effective, and there is concern that widespread use of primary prophylaxis will exacerbate the problem of drug resistance and drug-drug interactions. Therefore, we do not recommend primary prophylaxis for oropharyngeal or esophageal candidiasis. Whereas primary prophylaxis for Candida is rarely provided, secondary prophylaxis can be given if patients have multiple and frequent recurrences of oropharyngeal or esophageal candidiasis. Oral fluconazole 50–100 mg/day or 150 mg once-weekly is effective prophylaxis against recurrent oropharyngeal and esophageal candidiasis in patients with azole-sensitive disease.25 Caspofungin has also been found effective in such cases but must be given intravenously.38
Treatment for GI CMV disease is limited to intravenous therapy with ganciclovir, foscarnet, and cidofovir. Valganciclovir has not been studied as primary therapy for enteric CMV disease.42 A number of trials using intravenous ganciclovir in HIV-infected patients with GI CMV disease have demonstrated clinical improvement in ∼75% of patients.43,44 Open-label trials of foscarnet have yielded comparable results.45 The only placebo-controlled trial of ganciclovir, which evaluated colitis, found no clinically significant differences between the treatment groups probably because the treatment period was only 2 weeks.46 The only randomized controlled study comparing ganciclovir to foscarnet for therapy of CMV esophagitis found no difference between the agents regarding clinical activity, but there was more toxicity with foscarnet.44 Marked endoscopic improvement was observed in 73% of the foscarnet-treated patients and 70% of the ganciclovir-treated patients. The symptomatic response was also similar for the two treatments: 82% of patients who received foscarnet and 80% of those treated with ganciclovir had a complete or at least a good clinical response. A randomized trial comparing ganciclovir to foscarnet in 48 AIDS patients with GI CMV disease found similar clinical efficacy (73%) regardless of the location of the disease (esophagus versus colon), with endoscopic improvement documented in more than 80%.47 The time to progression of disease was similar (13–16 weeks) despite the use of maintenance therapy. Side effects occurred in half the patients in both groups. Mönkemüller and Wilcox described one case of CMV esophagitis that resolved after initiation of HAART without specific antiCMV therapy.48 That report suggested that immune reconstitution with HAART can be associated with clearance of an opportunistic GI infection.
The decision to use either ganciclovir or foscarnet for GI CMV disease in AIDS should be based on the experience of the physician and the differing toxicities of each agent. The efficacy, tolerability, and cost of ganciclovir have established it as first-line therapy for GI CMV disease in AIDS. Our current policy for the therapy of GI CMV disease is to administer intravenous ganciclovir, assuming there are no major contraindications to this agent such as neutropenia or thrombocytopenia. The usual induction dose is 10–15 mg/kg administered twice a day for 3–4 weeks. In our experience, esophageal disease tends to respond more rapidly than does colonic disease. The response to therapy is judged by alleviation of symptoms and improved endoscopic findings. Ophthalmologic examination is mandatory at the time of diagnosis in all patients to exclude retinal disease. If retinal disease is absent and a complete symptomatic and endoscopic response is documented following induction therapy, we stop therapy and observe for recurrent symptoms. For patients with persistently low CD4+ T-lymphocyte counts, the relapse rate for esophageal and colonic disease is similar (30–50%).43,44,47 Initiation of HAART therapy, or improving existing ART, plays an important role for prevention of recurrence. Endoscopic reexamination with biopsy of any mucosal abnormalities is important for patients with persistent symptoms following therapy. For those with frequent relapses of GI disease, long-term once-daily maintenance intravenous administration is appropriate. Although there are no reported data regarding the efficacy of oral valganciclovir for either maintenance therapy or treatment of acute GI disease, valganciclovir is likely to be effective in this setting and is a reasonable choice for treatment.49 Failure to respond to ganciclovir may be the result of low serum levels50 or drug resistance.51
For patients with major contraindications or failure to respond to ganciclovir, foscarnet is usually effective.47,52 The recommended dosing schedule is 90 mg/kg IV bid daily for 14–21 days. Salzberger et al53 demonstrated in an open-label, randomized trial that a frequency reduction of fosarnet from 7 to 5 days a week for 3 weeks was associated with equal response and fewer side effects than using the medication daily for 21 days. Combination therapy of foscarnet (90 mg/kg bid daily) and ganciclovir (5 mg/kg bid daily)54 may be as effective for ganciclovir failures. A small study evaluated the safety of induction and maintenance therapy alternating ganciclovir (5 mg/kg every other day) and foscarnet (120 mg/kg every other day). There appears to be little benefit with their approach. The efficacy and incidence of side effects seemed to be equivalent to daily monotherapy or dual therapy.54
Randomized placebo-controlled studies of oral ganciclovir for primary prophylaxis have demonstrated a reduction in the incidence of retinal involvement.55 No definitive data exist on the effectiveness of primary prophylaxis for decreasing GI CMV disease in HIV-infected patients. Restoration of immune function with HAART is the primary strategy for prophylaxis.
For the patient with mild to moderate HSV esophagitis who is able to tolerate pills, oral administration of acyclovir 15–30 mg kg−1 day−1 is effective.56 The drug is usually given in a dose of 400 mg PO five times a day for 2 weeks. Because absorption of oral acyclovir is inconsistent and may be as low as less than 30%,57 valacyclovir 500–1000 mg bid PO has become the treatment of choice, especially for patients with more severe disease. Oral famciclovir is also highly effective against HSV. Intravenous administration of acyclovir should be applied when severe odynophagia limits oral intake or when the patient has not responded to high-dose oral therapy. Several studies have confirmed the safety and efficacy of intravenous foscarnet (40 mg/kg every 12 h) for treating HSV disease and supported the utility of this agent as maintenance therapy for delaying recurrences.58 Primary prophylaxis is not currently recommended; secondary prophylaxis with valacyclovir (500 to 1000 mg/day) is usually provided to patients with frequent relapses of oropharyngeal or esophageal disease. Drug resistance is rare.59
Prospective studies of idiopathic esophageal ulcers have documented healing rates of more than 90% with oral corticosteroids.60 The regimen most commonly employed is prednisone 40 mg/day tapering 10 mg/week for a 1-month treatment course.60 Shorter courses of therapy may be effective for small ulcers. Although beneficial, intralesional injection of corticosteroids should be considered as second-line therapy. The side effects of corticosteroids are well recognized; patients with AIDS may be more likely to develop CMV disease while on therapy.61 Because oropharyngeal and esophageal candidiasis may complicate steroid use and confuse the therapeutic response, we routinely use short courses of azole therapy while the patients are receiving prednisone. The response to corticosteroids is rapid, with most patients experiencing significant pain relief within days. Although not as well studied, thalidomide appears to be highly effective for IEU.62,63 In doses of 200–300 mg/day, thalidomide has been documented to result in a clinical response and endoscopic cure in more than 90% of treated patients.63 Thalidomide is well tolerated, with the main side effect being somnolence; administration of the drug at bedtime tends to overcome this problem. Peripheral neuropathy and skin rash are infrequent side effects. The major fear with thalidomide is inadvertent use during the first trimester of pregnancy, which consistently results in severe birth defects. Therefore, most physicians do not prescribe this agent for women of childbearing age unless the patient is surgically sterile. Without improvement of immune function, the relapse rate of IEU is ∼40–50% regardless of the initial therapy.60,63
INFECTIONS OF THE STOMACH
Symptomatic and clinically significant opportunistic gastric infections are uncommon in patients with HIV infection. While a number of opportunistic pathogens have been reported to infect the stomach in these patients, including cryptosporidia, Toxoplasma gondii, Leishmania, Pneumocystis carinii, Treponema pallidium, Cryptococcus neoformans, the most common is CMV.64,65 All these infections may be asymptomatic or result in nausea and vomiting. Most present in the setting of disseminated disease and are associated with systemic symptoms (malaise, weakness, fever). Clues to the diagnosis may be found outside the GI tract. Epigastric pain or GI bleeding occur more frequently in the setting of ulcerative lesions such as peptic ulcer disease and CMV. The main diagnostic test for patients with these symptoms is upper endoscopy with biopsies.
Although earlier studies report that the prevalence of peptic ulcer disease and Helicobacter pylori infection were common in patients with HIV infection, subsequent studies performed since then have found that the prevalence of peptic ulcer disease and H. pylori is lower in HIV-infected patients, especially those with lower CD4 lymphocyte counts.66,67 Possible explanations for this phenomenon include hypochlorhydria, antibiotic use, and inadequate mucosal inflammatory response.68 Varsky et al studied 497 HIV-positive patients with upper digestive tract symptoms.69 The investigators found that 5% of these patients had gastroduodenal ulcers (GDUs). Helicobacter pylori was detected in only 31% of patients with GDUs, whereas CMV was detected in 50% of these patients. In HIV-infected patients with chronic active gastritis without a GDU, other organisms such as Cryptosporidium and CMV were more prevalent than H. pylori. Based on their findings it is recommended that endoscopic biopsies be performed to search for opportunistic pathogens in AIDS patients with upper digestive symptoms. Most studies from Europe, Australia, and the United States have also found a low incidence of H. pylori in HIV-infected patients. In Italy, Cacciarelli et al found that the prevalence of H. pylori in HIV-positive patients with a CD4+ T-lymphocyte count of less than 200 cells per cubic millimeter was significantly less than that of HIV-negative patients.66 The authors also found that the number of peptic ulcers was significantly less in HIV-infected patients. In New York, Marano et al found H. pylori in only 15.9% of symptomatic HIV-infected patients undergoing upper endoscopy, despite the presence of chronic active gastritis in 94.5% of them.70 In Australia, Edwards et al found that the prevalence of H. pylori was 3% in patients with AIDS, compared to 22% in non-HIV-infected patients.71 Mönkemüller et al reported that the incidence of opportunistic GI infections has declined, most likely secondary to the use of HAART.1 During the same period they noted a rise in the number of nonopportunistic GI disorders in these patients, including peptic ulcer disease.1
ABDOMINAL PAIN
The diagnosis of abdominal pain can be a challenge given the broad spectrum of potential causes, including both opportunistic and nonopportunistic disorders.72,73 In most cases a carefully performed history and physical examination in conjunction with the CD4+ T-lymphocyte count can help narrow the differential diagnosis. A thorough systematic evaluation is necessary to avoid overlooking potentially life-threatening conditions, such as hollow viscus perforation, appendicitis, intestinal obstruction, pancreatitis, and toxic megacolon. It is also important to remember that multiple conditions associated with ongoing, chronic abdominal pain such as CMV enteritis and non-Hodgkin lymphoma (NHL) can suddenly result in an acute abdomen due to intestinal perforation and obstruction.73 Treatment of abdominal pain is directed by the underlying etiology.
Etiology
A cause of abdominal pain can be found in most HIV-infected patients, and treatment should be tailored accordingly. The most common causes of chronic abdominal pain in patients with advanced HIV disease (i.e., CD4+ T-lymphocyte count <100 cells per μL) are disseminated Mycobacterium avium complex (MAC), intestinal CMV disease, and neoplasms such as NHL and Kaposi sarcoma (KS) (Table 77-2).
Table 77-2 Etiology of Abdominal Pain in Patients with HIV Infection
| Epigastric |
| Acute and chronic pancreatitis (CMV, drug-induced, TMP-SMX) |
| Peptic ulcer disease (gastric ulcer, duodenal ulcer) |
| Gastritis (Helicobacter pylori, CMV, Cryptococcus neoformans, mucormycosis, cryptosporidia, Leishmania donovani) |
| Duodenitis (CMV, L. donovani, Cryptococcus neoformans, Cryptosporidium parvum) |
| Nonulcer dyspepsia) |
| Periumbilical |
| Enteritis (viral: CMV, rotavirus, astrovirus, picorna virus, coronavirus; bacteria: Salmonella, MAC, M. tuberculosis; protozoa: Isospora belli, cryptosporidia, microsporidia, Cyclospora cayetanensis, Giardia lamblia; fungi: Cryptococcus neoformans (duodenum) |
| Lymphoma |
| Right Upper Quadrant |
| Cholecystitis (gallstones and acalculous). Acalculous most frequently secondary to infections: CMV, Isospora belli, Candida, cryptosporidia, microsporidia, Salmonella spp., Campylobacter fetus |
| AIDS-cholangiopathy (CMV, MAC, Salmonella spp., Enterobacter spp., cryptosporidia, microsporidia, Cyclospora cayetanensis) |
| Shingles (varicella zoster) |
| Hepatitis (A,B,C,D, CMV, EBV) |
| Perihepatitis (Chlamydia trachomatis, Neisseria gonorrhoeae), Bartonella |
| Left Upper Quadrant |
| Splenic abscess |
| Pancreatic abscess (TB) |
| Pancreatitis (ddI, ddC, pentamidine, TMP-SMX, Cryptosporidium, Campylobacter, CMV, HIV) |
| Shingles |
| Right Lower Quadrant |
| Lymphoma |
| Appendicitis |
| Inflammatory bowel disease |
| Pelvic inflammatory disease |
| Ectopic pregnancy |
| Left Lower Quadrant |
| Colitis (infectious: viral: CMV, rotavirus, astrovirus, picobirna virus, coronavirus, adenovirus, HSV; bacterial: Shigella, Campylobacter, Salmonella, Clostridium difficile, Mycobacterium avium, Mycobacterium tuberculosis, Bartonella henselae, Aeromonas hydrophila, protozoa: Entamoeba histolytica, Isospora belli, Cryptosporidium, Toxoplasma gondii, Schistosoma mansonii, Dientamoeba fragilis, Blastocystis hominis, microsporidia; fungi: Histoplasma capsulatum, Candida albicans, Cryptococcus neoformans, Pneumocystis carinii neoplastic: Kaposi sarcoma (Herpesvirus 8), non-Hodgkin lymphoma, idiopathic, drug-induced (acyclovir) |
| IBD (idiopathic, also can occur in association with KS) |
| Diverticulitis |
| IBS |
| Pelvic inflammatory disease |
| Ectopic pregnancy |
| Flanks |
| Kidney stones (drug-related: indinavir) |
| Pyelonephritis |
| Retroperitoneal lymphadenopathy (non-Hodgkin lymphoma, angioimmunoblastic lymphadenopathy, tuberculosis, MAC) |
| Suprapubic |
| Cervical cancer |
| Pelvic inflammatory disease |
| Ectopic pregnancy |
| Not localized to specific area: |
| Toxic megacolon (CMV, C. difficile, Cryptosporidium, KS) |
| Colonic perforation (CMV, histoplasmosis, idiopathic, diverticulum, neoplasm) |
| Peritonitis |
| Ileal perforation |
| Neoplasm (lymphoma, Kaposi sarcoma) |
| Adrenal failure |
| Diffuse |
| Peritonitis (TB, CMV, Toxoplasma gondii, Cryptococcus neoformans, Histoplasma capsulatum) |
| Bowel perforation |
| Intraabdominal lymphadenopathy (lymphoma, KS, MAC, TB, angioimmunoblastic lymphadenopathy, bartonellosis) |
| Adrenal failure-adrenalitis |
| Mesenteric fibrosis |
| Omental fibrosis (H. capsulatum) |
| IBS |
HIV-infected patients are at similar risk of suffering from diseases and abdominal pain due to the same etiologies as nonimmunocompromised patients. This list highlights some conditions seen frequently in HIV-infected patients.
Microsporidia: Enterocytozoon bieneusi, Encephalocytozoon (Septata) intestinalis, Encephalocytozoon cuniculi.
EBV, Epstein–Barr virus; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; KS, Kaposi sarcoma; MAC, Mycobacterium avium complex.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree