Author
No. of patients (n)
Follow-up (months)
Remission rate (%)
Overnight dexa
Bochicchio et al. [41]
668
Mean 24; median 13
76.3
Chee et al. [56]
61
Median 88
78.7 (at 12 months)
67.2 (at 14–211 months)
Hofmann et al. [65]
426
369 (adenomectomy)
73.2 ± 43.2
(range, 13–207)
75.9
UFC
Fomekong et al. [59]
40
Mean 84 ± 44
80
Kim et al. [47]
54
Mean 50.7
70.4
Patil et al. [52]
215
Mean 45
85.6
Prevedello et al. [64]
167
Mean 39 (range, 6–157)
88.6 (100 % microadenoma)
mSC + UFC
Acebes et al. [57]
44
Mean 49 (range, 19–102)
89
Invitti et al. [66]
288
Not available (for n = 288)
Range 6–180 (for n = 129)
69
Jagannathan et al. [68]
261
Mean 84 (range, 12–215)
96.5
Salenave et al. [62]
54
Mean 19.9 ± 22.7 (range, 1–89)
66.5
Swearingen et al. [49]
161
154 (TSS)
Mean 104.4; median 96 (range, 12–240)
90 (microadenoma)
65 (macroadenoma)
85 (total)
Valassi et al. [43]
620
Range, 1–300
70.5
Yap et al. [51]
97
Mean 92 (range, 6–348)
68.5 (immediate)
Morning SC
Bou Khalil et al. [58]
127
Mean 48.8 (range, 3.7–148.7)
79.5 (early)
62.9 (maintained)
Carrasco et al. [42]
68
Range, 6–12
74
Esposito et al. [18]
40
Mean 33 (minimum 14)
79.5
Flitsch et al. [46]
147
61
93
Hassan-Smith et al. [60]
80
Median 55.2
83 (early remission)
72 (cure)
Rees et al. [61]
54
Median 72 (range, 6–252)
77
Storr et al. [63]
155 (microadenoma)
28 (macroadenoma)
Not available
Overall 61 (microadenoma)
32 (macroadenoma)
Trainer et al. [67]
48
Median 40 (range, 15–70)
42
SC + UFC + dexa suppression
Atkinson et al. [21]
63
Mean 115.2 (range, 1–252)
71.4 (early)
56 (overall)
Bakiri et al. [89]
50
Median 71.5 (range 21–219)
72
Barbetta et al. [90]
68
Median 57.5 (range, 12–252)
68 (early)
79 (persistent)
Jehle et al. [87]
193
Mean 57.6 ± 42 (range, 8.4–148.8)
80.8 (overall)
Alwani et al. [17]
79
Median 84 (range, 6–197)
65 (immediate)
Shimon et al. [91]
74
Mean 50.4 ± 34.8
78
An overall remission rate in 74 studies published between 1976 and 2014 [2], involving 6091 patients CD after TSS was reportedly 25–100 %, (mean 77.7 % and median 78.2 %). Recurrence rates ranged from 0 to 65.5 % (mean 13.4 % and median 10.6 %). This review included studies that overlapped significantly with those studies analyzed by Petersenn et al. [23]. Similar to previous data, the studies included were heterogeneous with a wide number of patients reported (range 6–668) and large variations in follow-up duration (Table 1). Furthermore, the criteria used to define remission and recurrence were not uniformly reported.
A subanalysis of studies with 30 or more patients and a minimum mean/median follow-up of 6 months reported that the percentage of failed pituitary surgeries for CD ranged between 5.7and 63 % with a mean of 31.4 % and median of 29.4 % [2]. In studies that further stratified results by adenoma size (micro vs. macro) the mean rates of remission were 85 % for microadenomas and 58 % for macroadenomas (Table 1) [18, 41–43, 46, 47, 49, 51, 52, 56–68].
Serial Serum Cortisol and ACTH in the Immediate Postoperative Period
In one study, Swearingen et al. looked at factors associated with remission and determined cure by using both fasting serum cortisol levels less than 138 nmol/L and UFC less than 55 nmol/day [49]. The 5-year cure rate for patients was lower than the 10-year one: 96 % for microadenomas, 96 % for macroadenomas vs. 93 % and 55 %, respectively.
In other studies, a serum cortisol of 50 nmol/L (1.8 μg/dL at ODST after surgery) and normal 24 h UFC was most frequently used to define remission [2]. Overall, patients with serum cortisol levels of <2 μg/dL in the immediate postoperative period achieved long-term remission at 10 years in approximately 90 % of cases [5, 15, 18, 21, 45, 51, 56, 61, 69, 70].
In a single-center study [71] in 52 patients with CD followed over a minimum of 6 years, early postoperative cortisol <2 μg/dL and ACTH < 5 pg/mL was a sensitive predictor of remission. The positive predictive value (PPV) for remission with postoperative nadir cortisol <2 μg/dL and ACTH < 5 pg/mL was 100 % (p < 0.005). The PPV for non-remission of ACTH > 15 pg/mL was 87.5 %. Interestingly, no patients with postoperative cortisol >10 μg/dL were found to have delayed remission. While this study found a lower cutoff value for ACTH and cortisol (<5 pg/mL and <2 μg/dL, respectively) than other studies to be highly predictive of remission, no level predicted the lack of recurrence. The addition of ACTH to cortisol measurements might increase accuracy of remission assessments [72].
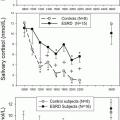
Late-Night Salivary Cortisol
In another single-center study that included 164 surgical CD patients, LNSC [40] had a 94 % sensitivity and 80 % specificity for remission at a cut off of 1.9 nmol/L within 3 months of TSS. A nadir morning serum cortisol of <5 μg/dL and nadir 24 h UFC of <23 μg was used to define remission, in these patients. Recurrence was established with LNSC at a cutoff of 7.4 nmol/L (75 % sensitivity and 95 % specificity) and 1.6-fold above normal 24 h UFC (68 % sensitivity and 100 % specificity), respectively, at a median follow-up of 53.5 months .
Delayed Remission After Surgery
Hormonal assessment in the immediate postoperative period, in rare cases, may be misleading in a subset of patients after TSS for CD because of delayed remission. A retrospective review of 620 patients who underwent TSS for CD between 1982 and 2007 in two large centers [43] classified outcomes into three groups based on the postoperative pattern of cortisol testing: IC for immediate control, NC for no control, and DC for delayed control. The IC group had a 70.5 % rate (437 of 620 patients) of hypocortisolism and/or cortisol normalization throughout the postoperative follow-up period. The NC group reported 23.9 % (148 of the 620 patients) with persistent hypercortisolism, while the DC group reported 5.6 % (35 of 620 patients) with early elevated or normal UFC levels and developed delayed and persistent cortisol decrease after an average of 38 ± 50 days [43].
Degree of Tumor Invasiveness and Remission
Shin et al. studied 49 patients who underwent EEA resection at a single institution over an 11-year period. The endocrinologic remission rates were analyzed according to degree of invasiveness, by Knosp score [35]. The Knosp score (ranging from 0 to 4) is based on the tumors relationship, as seen on preoperative MRI, to the cavernous segment of the internal carotid artery (ICA) [73]. In this study, the initial remission rate (36 h to 2 weeks postoperatively) was 79.6 % and was 70 % in patients with a mean follow-up of 37.5 ± 4.6 months. An initial remission rate of 80 % was reported in MRI negative adenomas, 84.8 % among noninvasive/minimally invasive adenomas and 50 % among invasive adenomas.
This further highlights the challenges of treating patients with invasive tumors. Interestingly, preoperative UFC levels were not significantly different with respect to degree of tumor invasiveness and had no significant effect on remission rate in this series. However, a higher preoperative ACTH level was associated with a higher degree of invasiveness.
Timeline to HPA Recovery After Remission of CD
Hypothalamic–pituitary–adrenal axis recovery after remission of CD is variable, between 13 and 25 months [38, 44, 74–76]. In a study of 91 patients with CS, 54 with CD [77], CD patients were divided into three groups: group 1, patients with normal postoperative pituitary function and no recurrence; group 2, patients with later recurrence after successful surgery; and group 3, patients who displayed postoperative additional anterior and posterior pituitary insufficiencies, presumably because of a more radical surgical approach. Those cured were defined by the development of postoperative tertiary AI requiring glucocorticoid replacement therapy. Recurrence occurred between 2.4 and 14.4 years after surgery (mean, 7.2 ± 4.6 years). The three CD groups were not different with respect to age, preoperative BMI, male-to-female ratio, duration of symptoms, or other biochemical parameters [77]. The authors hypothesized that this stratification would enable them to identify if normal pituitary gland tissue damage, as a result of surgery, significantly influenced HPA recovery. Plasma cortisol and ACTH, UFC, and salivary cortisol were all studied. A subgroup analysis showed that the probability of recovery at 5 years was 71 % in group 1 and 100 % in group 2. Group 3 patients had the poorest rate of recovery. Only in group 1, the probability to recovery of adrenal function was associated with younger age independent of sex, BMI, duration of symptoms, basal cortisol, and basal ACTH levels. The mean age of patients experiencing recovery was 37 years of age at the time of surgery, compared with 48 in patients without recovery.
The long-term occurrence of hypocortisolism after TSS has been hypothesized to be associated with the number of Crook’s cells present [76]. Similarly, Saeger et al. [78] associated Crook’s cell count and severity of glucocorticoid excess in CD. Crooke’s hyaline change was first described in 1935 in the normal anterior pituitary surrounding an ACTH-secreting adenoma. Non-neoplastic corticotrophs have increased eosinophilic cytoplasm filled with perinuclear cytokeratin while the adenoma itself does not. The cause of Crooke’s hyaline change is uncertain, but it is related to increased glucocorticoid or cortisol levels [79].
Another factor at play may be the duration of exogenous glucorticoid received by the patient and its effects on the adrenal gland. Sacre et al. [80] analyzed AI rates following pharmacologic glucocorticoid treatment for various inflammatory disorders and found that cumulative dose and exposure time were independent predictors of AI. Berr et al. concluded that the recovery of corticotroph function is due to residual tumor cell clusters rather than by hypothalamic CRH-mediated stimulation on normal corticotroph cells [77]. After multivariate analysis, this study identified younger patient age as an independent significant factor influencing HPA recovery in patients with CD. The preoperative degree of hypercortisolism and postoperative glucocorticoid replacement doses did not seem to be relevant .
Cushing’s disease also has accompanying disturbances in growth hormone (GH) and prolactin (PRL) secretion [81–84]. A small study compared eight adults (five females and three males) with CD in remission with eight healthy patients matched for gender, BMI, and age. Remission was established by the absence of signs and symptoms during long-term follow-up of 8.2 ± 1.7 years, normalized 24 h UFC, and suppression of morning plasma cortisol concentration below 0.10 μmol/L after the administration of 1 mg dexamethasone, orally, at 2300 h (at yearly visits in an out-patient clinic). Before TSS, ACTH and cortisol levels were found to have elevated basal rates, augmented secretory pulse amplitudes, blunted or absent diurnal variation characteristics, and a loss of orderly secretory patterns [85, 86] but the 24 h secretion properties of ACTH, cortisol are normalized after clinically successful TSS [84]. Physiological recovery was determined by total secretory activity (pulsatile and non-pulsatile), diurnal rhythmicity, and the orderliness of the release process. Further studies are needed to determine if all physiological characteristics of ACTH, cortisol, GH, and PRL secretion can be consistently normalized after TSS in CD .
How to Diagnose Early Recurrent Cushing’s Disease
Recurrence Rates
Definitions of recurrence are poorly characterized, heterogeneous, and furthermore, infrequently reported in the literature. The general criteria used to define recurrence include a combination of a relapse of symptoms, clinical features, and/or biochemical confirmation .
Some studies defined recurrence just on the basis of questionnaire response and results of routine endocrine reevaluation locally, without independent repeated assay in the initial center [42, 43, 47, 48, 51, 52, 57–62, 70, 87, 88]. The most frequently utilized biochemical tests to detect recurrence are 24 h UFC and 1 mg DST. However, measuring LNSC has been shown to be sensitive and is becoming more commonly used [40–42]. In the literature, some studies used the same criteria to determine remission and recurrence while others used a separate criteria for each [23]. Urine-free cortisol testing was used either alone or more commonly in combination with serum cortisol and/or LDDST as an endpoint for establishing recurrence (Table 2) [42, 43, 47, 48, 51, 52, 57–62, 70, 87, 88].
Table 2
Recurrence: clinical and biochemical assessments
Author | No. of patients (n) | Follow-up (months) | Recurrence rate (%) | |
|---|---|---|---|---|
Morning cortisol + UFC | Bou Khalil et al. [58] | 127 | Median 50.4 (range, 7–99) | 20.8 (mild or overt) |
mSC + UFC + dexa | Carrasco et al. [42] | 68 | Mean 51 ± 30 (range, 9–90) | 14.3 |
Jehle et al. [87] | 193 | Mean 57.6 ± 42 (range, 8.4–148.8) | 13.5 | |
Kim et al. [47] | 54 | Median 50.7 | 32.4 (at 5 years) 54.6 (at 10 years) | |
UFC | Fomekong et al. [59] | 40 | Mean 95 ± 35 | 9.4 (overall) 14 (microadenoma) 0 (macroadenoma) |
Guilhaume et al. [88] | 64 | Range, 24–36 | 14.28 | |
Patil et al. [52] | 215 | Mean 45 | 17.4 | |
UFC + dexa | Hassan-Smith et al. [60] | 80 | Median 55.2 | 11 |
Pereira et al. [70] | 78 | Median 84 Median 174 (range, 120–288) | 9 (n = 5 of 56) 16.7 (n = 4 of 24) | |
Sonino et al. [48] | 162 | Median 84 | Not stated | |
Yap et al. [51] | 89 | Mean 36.3 | 11.5 | |
mSC + UFC | Acebes et al. [57] | 44 | Mean 25 (range, 2–102) | 7.7 |
Rees et al. [61] | 54 | Median 17 (range, 6–50) | 5.1 | |
Salenave et al. [62] | 54 | Mean 19.9 ± 22.7 (range, 1–89) | 19.5 | |
Valassi et al. [43] | 620 | Median 66 | 13 (total) |
Recurrence is reportedly lowest when a combination of LDDST and UFC was used as biochemical endpoints regardless of whether serum cortisol and/or clinical parameters were also included in the assessment of recurrence [17, 21, 87, 89–91]. Overall, recurrence rates in all studies, regardless of methods used to determine recurrence, were slightly less in the group of patients with microadenomas vs. macroadenomas, 13.4 % vs. 17.6 %, respectively, but not statistically significant [23]. The duration of follow-up ranged from 13 to 96 months [23], but follow-up time did not predict rate of recurrence. The limitations in interpreting these resultant conclusions need to be taken into account given the small number of studies involved. If one further analyzes the data for the studies that reported rates of recurrence for both microadenomas and macroadenomas, the mean rate of recurrence for microadenomas was 10.9 % (four studies) and 23.6 % (two studies) for macroadenomas [55, 63, 92]. Interestingly, studies that only used biochemical tests to determine overall recurrence rates reported a relatively similar rate (15.7 %) vs. the ones that used both clinical and biochemical endpoints to determine rate of recurrence reported 14.4 % [23]. The overall calculated recurrence rate was 15.2 % and meantime to recurrence (in the 23 studies where was reported) was 50.8 months (range 3–158 months ) [23].
Biochemical Testing Timeline
Different timelines of change from normal to abnormal in some biochemical tests are also interesting. A study that looked at sequential alterations over time after surgery in 101 patients [58] found that 21 (20.8 %) presented with recurrence, ‘mild’ or ‘overt’, during long-term follow-up (median 50.4 months, range 7–99). Interestingly, vasopressin analogs and CRH tests were eventually positive in 85 and 93 % of all patients who experienced disease recurrence. Recurrence occurred less frequently and later in patients with early AI compared with patients with normal cortisol after surgery. Increase in LNSC occurred in a mean time of 38.2 months, while UFC elevation was observed at 50.6 months; however, a positive response to vasopressin analogs or CRH preceded the increase in midnight cortisol or UFC in 71 % and 64 % of patients, respectively.
Combined Biochemical Testing
Coupled dexamethasone desmopressin test (CDDT) has been also suggested as good predictor of recurrence of CD after surgery [93]. In a small study (38 patients) followed for a median of 60 months, CDDT became positive in eight of ten patients with recurrence 6–60 months before classical markers of CD. Similar to other studies, AI did not ensure lack of recurrence: six patients with immediate postsurgical corticotroph deficiency presented with recurrence; however, all patients had abnormal CDDT positivity during the 3 years after surgery with recurrence 6–60 months after CDDT positivity. CDDT has been considered an early predictor of recurrence of CD and could be of particular interest in the first 3 years after surgery, by selecting patients at high risk of recurrence despite falsely reassuring classical hormonal markers [93]. However, a comparison with LNSC in predicting recurrence remains to be determined.
Degree of Tumor Invasiveness and Recurrence
The degree of tumor invasiveness has also been shown to play a role in potentially influencing recurrence rates [35].
Impact of Delayed Remission on Recurrence
In a large study [43] (described in more detail in the remission section), 35 of 620 patients (5.6 %) had delayed control defined as early elevated or normal UFC levels and developed a delayed and persistent cortisol decrease after an average of 38 ± 50 postoperative days. These patients with delayed remission vs. those with immediate control of CD after TSS seem to have significantly higher cumulative rate of recurrence at 4.5 years, 43 % vs. 14 %, respectively over a median of 66 months after TSS with a total recurrence rate of 13 % [43]. Criteria for recurrence in this particular study included at least two abnormal tests from the following four: elevated serum cortisol or 24-h UFC, abnormal ODST, here defined as cortisol >5 μg/dL (138 nmol/L), or abnormal serum cortisol during the combination of low-dose dexamethasone suppression test and ovine or human CRH stimulation test .
Assessment Criteria in Patients with Cushing’s Disease Treated with Medical Therapy
The assessment of CD remission after a patient is started on medical therapy is very complex and remains controversial, overall [2, 11]. Therapies with agents acting at the pituitary level (cabergoline, pasireotide), adrenal steroidogenesis inhibitors, and a glucocorticoid receptor blocker (mifepristone) are reviewed below, with a focus on biochemical markers and clinical improvements; mechanism of action of each drug, study design, and adverse events have been previously and extensively reviewed [25–29].
Biochemical Testing
24-Hour Urine-Free Cortisol
A retrospective analysis of 137 patients with clinical conditions suggestive of hypercortisolism, 38 with confirmed CS diagnosis and 99 without, found that UFC revealed both a combined higher positive and a lower negative likelihood ratio for diagnosing CS among first-line tests (10.7 and 0.03, respectively) [94]. Computing a receiver operating characteristic (ROC)-contrast analysis to compare the power of each single test with that of the others, alone or combined (DST + LNSC , DST + UFC and LNSC + UFC), or with that of all the tests together (DST + LNSC + UFC), UFC assay was at least as good as all the other possible combinations. The different results noted compared with other studies could be related to the liquid chromatography–mass spectrometry/ mass spectrometry (LC-MS/MS) method used for UFC. In that particular study, LNSC was measured by radio-immunometric method and serum cortisol by chemiluminescence immunoassay [94].
The reliability and reproducibility of UFC are both very important [94, 95]. Newer methods such as LC-MS/MS have revealed that the analytical performance of UFC is better than urinary cortisol:cortisone ratio in detecting CS.
Intra-patient UFC variability at diagnosis is a well-known caveat; large studies have shown up to 50 % variability [96] and overall variability in mUFC increased as UFC levels increased. However, there were no correlations between UFC and clinical features of hypercortisolism. The assay used is even more important at potentially lower values of UFC when determining remission or recurrence. Most clinical studies looking at the effects of medical therapies have measured UFC during treatment; furthermore, new clinical guidelines [14] emphasize that despite some caveats, UFC is a good marker to monitor therapy response. One important exception represents treatment with a glucocorticoid receptor blocker, in which case UFC is not reliable and monitoring has to rely solely on clinical grounds and other biochemical assessments such as glucose for example.
While for diagnosis, at least two 24-h UFC are recommended [10], the number of UFCs needed to ensure correct assessment for remission is still unclear. The UFC variability with regards to medical treatment is largely unknown. A summary of studies using UFC as marker for biochemical response on medical therapy can be found in Table 3 [9, 93, 97–108].
Table 3
Summary of biochemical and clinical markers identified as endpoints for response to medical therapy in Cushing’s disease and Cushing’s syndrome
Author | Study design | Follow-up (months) | Medication | Patients (n) | Definition of response | |||
|---|---|---|---|---|---|---|---|---|
24 h UFC | Late-night SC | Overnight Dexa | Serum Cortisol | |||||
Boscaro and Arnaldi [9] | Prospective | 15 days | Pasireotide | 39 | Normal | |||
Colao et al. [100]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||