Diffuse Large B-Cell Lympho
Lymphomas are a malignancy arising from lymphoid cells, with more than 60 distinct variants identified by the World Health Organization (WHO). Lymphomas can originate in B cells, T cells, or natural killer cells, and are broadly categorized into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). Within NHL, further differentiation is made based on clinical presentation and histology. About 85% of lymphomas in the United States and Western Europe are of B-cell origin. Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of NHL in North America and the focus of this chapter. The majority of the information in this chapter is applicable to DLBCL-NOS (not otherwise specified). Short discussions of other subtypes of diffuse large B-cell lymphoma and of other lymphomas of large B cells are included at the end of this chapter.
EPIDEMIOLOGY
DLBCL is a B-cell lymphoma characterized by malignant proliferations of lymphocytes at various stages during the normal B-cell maturation process and accounts for 30%-40% of all NHL cases. The annual incidence is approximately 16.5 per 100,000 people per year, with a slightly higher incidence in men compared to women (SEER). Comparing incidence based on race, Caucasians have the highest and American Indians/Alaskan Natives the lowest (SEER). The median age of diagnosis is 67 years old.
In a majority of patients, no clear risk factor can be identified; although the proportion of Epstein-Barr virus associated DLBCL is greater in the elderly, suggesting a possible viral connection. HIV strongly increases the risk of lymphoma, with DLBCL being the most common HIV-associated lymphoid malignancy. The underlying pathophysiology is likely due to chronic antigenic stimulation causing polyclonal B-cell expansion and then subsequent emergence of monoclonal B cells. Autoimmune rheumatologic diseases, such as Sjogren’s, lupus, and rheumatoid arthritis have also been associated with the development of DLBCL, especially in patients with detectable autoantibodies and substantial clinical involvement. In these diseases, chronic immune stimulation may promote lymphoma development although the role of immunosuppressive medication regimens is also being studied. Finally, a small number of patients present with histologic progression or transformation to DLBCL after a diagnosis of an indolent NHL, such as follicular lymphoma, or chronic lymphocytic leukemia. Transformation of chronic lymphocytic leukemia into diffuse large B-cell lymphoma is known as Richter’s transformation.
PATHOLOGY
Definitive diagnosis is made via excisional biopsy or core needle biopsy (fine needle aspiration is inadequate) with hematopathologic review of slides. Microscopic examination usually demonstrates a diffuse infiltrate of large lymphoid cells completely effacing the normal nodal architecture. The neoplastic cells are large lymphocytes with nuclei greater than twice the size of small lymphocyte nuclei, prominent nucleoli, and amphiphilic to basophilic cytoplasm. Histology can reveal centroblastic and immunoblastic cell types but these distinctions are not highly reproducible and do not have clinical implications. For most DLBCL subtypes, the cells express the pan-B cell markers CD19, CD20, and CD79a. CD5 is expressed in 5%–10% of cases and blastoid mantle cell lymphoma should be excluded in these cases by absence of the t(11;14). Overexpression of BCL6 is common.
CHARACTERISTICS
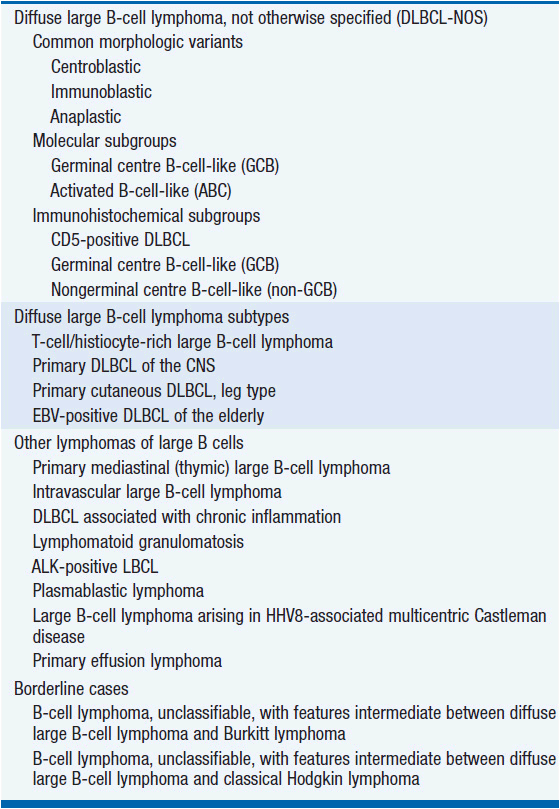
The WHO divides DLBCL into subtypes based on clinical, morphological, immunological, and genetic features (Table 31-1).
Pathogenic mutations commonly seen in DLBCL-NOS involve BCL-6, BCL-2, c-Myc, as well as genes in the NF-κB pathway.
Gene-expression profiling has divided DLBCL into distinct molecular subtypes: activated B-cell subtype (ABC), germinal-center B-cell subtype (GCB), type 3, and primary mediastinal B-cell lymphoma (PMBL). These subtypes are characterized by distinct clinical presentations, differential gene expression, and likely arise from B cells at varying stages of differentiation. A number of immunohistochemical algorithms have been reported to replicate profiling based subtype classification. Further studies are underway to determine the impact of these subtypes on therapy choice and outcome, and gene expression profiling is not currently used routinely.
DIAGNOSIS AND STAGING
DLBCL patients may present with symptoms of a rapidly enlarging lymph nodes, commonly in the neck or abdomen, sometimes accompanied by B symptoms of fevers, night sweats, and unintentional weight loss (Table 31-2). Up to 40% of patients will present with extranodal disease.
Staging is via the Ann Arbor staging system, which was originally developed for Hodgkin lymphoma (HL) (Table 31-3).
Approximately 27% will presents with stage I disease and 50% with advanced stage disease at diagnosis (SEER).
Initial evaluation of newly diagnosed DLBCL should include (NCCN) (1):
• Thorough history and physical examination with attention to nodal areas and to the liver and spleen
• B symptom inventory
• Performance status assessment
• International Prognostic Index score calculation (see Prognosis below)
• Laboratory studies: CBC with differential, comprehensive metabolic panel, LDH, uric acid, hepatitis B testing
• Imaging: CT of the chest/abdomen/pelvis with attenuation corrected PET or full diagnostic PET-CT
• Cardiac status: assessment of ejection fraction if anthracycline based chemotherapy regimen is planned
• Bone marrow biopsy with or without aspirate
• Pregnancy test
In selected cases, lumbar puncture (in patients with neurologic signs or symptoms or bone marrow involvement), HIV test, CNS imaging, and fertility discussions may also be useful (NCCN).
Functional imaging (FDG-PET) is used at diagnosis to accurately stage patients as well as early during the course of chemotherapy to risk stratify patients and guide treatment. It is nearly 100% sensitive for DLBCL when lymph nodes are above the size detection limit.
PROGNOSIS
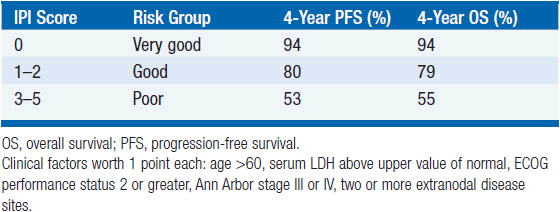
Prognosis is determined based on the International Prognostic Index (IPI) (2), a scoring system prognostic in the setting of rituximab-based chemotherapy regimens for event-free survival, progression-free survival, and overall survival in 2010 (3). It is based on five clinical factors (Table 31-4):
1. Age > 60
2. Serum LDH above upper value of normal
3. ECOG performance status 2 or greater
4. Ann Arbor stage III or IV
5. Two or more extranodal disease sites
The overall survival of patients at 4 years ranges from 94% for zero risk factors, down to 55% for patients with 3–5 risk factors.
Between 5% and 11% of patients with newly diagnosed diffuse large B-cell lymphoma will have concurrent translocations of myc and BCL-2. These cases are colloquially known as “double hit lymphomas” and have a poor prognosis with standard therapy.
Two recent studies have demonstrated that 20%–30% of newly diagnosed patients will have increased expression of myc and BCL-2 without a translocation. These patients have a response rate, progression-free survival, and overall survival intermediate between standard DLBCL and double hit lymphomas (4). The standard chemotherapy regimen of R-CHOP (Table 31-5) does not appear to provide satisfactory outcomes in this population and the therapeutic standard of care has not yet been established.
FRONT-LINE CHEMOTHERAPY
The mainstay of DLBCL treatment is combination chemotherapy. The current standard chemotherapy regimen is R-CHOP every 21 days (Table 31-5).
Rituximab is a chimeric monoclonal anti-CD20 IgG1 antibody that has demonstrated an additive effect when combined with CHOP to improve both progression-free and overall survival. One of the earliest reports of this survival benefit was from the Groupe d’Etude des Lymphomas de l’Adulte (GELA), which showed that in DLBCL patients over 60 years of age, regardless of IPI score at diagnosis, the addition of rituximab to CHOP improved complete remission and overall survival rates at 2 years by 10%–15% (5). Since then, several subsequent studies have confirmed this benefit in other DLBCL patient cohorts, affirming R-CHOP as first line treatment in DLBCL. R-CHOP given every 14 days has been compared to R-CHOP at 3-week intervals and is not superior.
 LIMITED-STAGE DIFFUSE LARGE B-CELL LYMPHOMA: STAGES I AND II
LIMITED-STAGE DIFFUSE LARGE B-CELL LYMPHOMA: STAGES I AND II
Patients with stage I or II bulky disease, defined as ≥10 cm in size, should receive 6 cycles of R-CHOP. Radiation therapy does not improve outcome over chemotherapy alone.
Patients with nonbulky disease, defined as <10 cm in size, can receive either 3 cycles of R-CHOP with radiation therapy or 6 cycles of R-CHOP without radiation therapy. The use of radiation therapy places the patient at a lifetime increased risk of second malignancy.
The decision to proceed with radiation therapy after chemotherapy completion depends on PET imaging results after completing R-CHOP. Biopsy should be considered in this setting with a positive PET scan:
• If there is a complete response (PET negative), then treatment is complete.
• Patients with a partial response (PET positive) should undergo biopsy. Those with persistent disease have the option of: (1) receiving radiation therapy to the PET positive site, (2) receiving high dose therapy with autologous stem-cell transplant, or (3) enrollment in a clinical trial. Repeat PET imaging is performed after completing the course of treatment with a repeat biopsy needed if scans return yet again positive.
• Patients with no response or progressive disease after the initial chemotherapy should receive treatment for refractory disease (see below).
Advanced-Stage Diffuse Large B-Cell Lymphoma: Stages III And IV
Patients with advanced stage disease should receive 6 cycles of R-CHOP. After the first 2–4 cycles of R-CHOP interim PET scans may sometimes be used to guide treatment; however, this approach has not been validated in prospective clinical trials.
• Those with a complete response (PET negative) should complete 6 cycles of R-CHOP and then have repeat PET scans. If the final scans continue to be negative; observation is indicated. If the final scans are positive, then treatment should be based on the algorithm for refractory disease below (after a biopsy has been obtained).
• Those with an interim partial response (PET positive) should complete 6 cycles of R-CHOP and have a final scan performed. Then the patient is managed as above for those with final scan negative or positive.
• For those without any response on interim restaging, patients should be treated for refractory disease. However, it is very rare that a PET scan is required to detect the failure of chemotherapy to produce a response. Refractory disease is nearly always a clinical diagnosis, often made by the patient.
In cases of poor left ventricular function, standard anthracyclines or anthracenediones cannot be used and alternative chemotherapy regimens and schedules are preferred (Table 31-6) (6).
TABLE 31-6 FIRST-LINE CHEMOTHERAPY IN PATIENTS WITH POOR LEFT VENTRICULAR FUNCTION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree