Differentiating Agents
An obvious feature of malignant cells is their failure to differentiate, and to acquire the histologic, biochemical, and functional features of the mature cells of the tissue from which they arise. Thus, leukemia cells resemble in appearance, surface markers, and molecular profile the more primitive normal progenitors of the myeloid or lymphoid series. Indeed it has been possible to isolate a small fraction of continuously self-renewing cells (called tumor stem cells) from frankly malignant tissues. These tumor cells are able to reproduce multiple differentiated cell lineages when appropriately stimulated by “differentiating” agents such as 5-azacytidine (see Chapter 1) or retinoids. The progression from mature cell phenotype to an undifferentiated malignancy is recapitulated in serial observations of chronic myelogenous leukemia and in experimental models of malignant transformation.
Research efforts have defined pathways responsible for a block in differentiation in malignant cells and have suggested strategies for pharmacologic intervention. Vitamin A and related retinoids were the first compounds to show differentiating effects in cell culture, and all-trans retinoic acid (ATRA) (Figure 9-1) was subsequently found to be highly effective in inducing remission in promyelocytic leukemia, a disease characterized by a translocation involving the retinoic acid receptor, RAR-alpha (1). Subsequently, other pathways have been exploited as targets for development of differentiating agents, including histone deacetylase (HDAC), DNA cytosine methyltransferase (CMT), and vitamin D signaling pathways. CMT is targeted by both 5-azacytidine and decitabine, as discussed in the section on antimetabolites, while HDAC inhibitors and vitamin A analogs are useful agents in peripheral T-cell lymphoma and acute promyelocytic leukemia (APL), respectively. Here we will consider four clinically useful agents that promote differentiation: ATRA and arsenic trioxide (ATO), both of which are effective in APL, and vorinostat and rhombedepsin, which are approved for treatment of cutaneous T-cell lymphoma (CTCL).
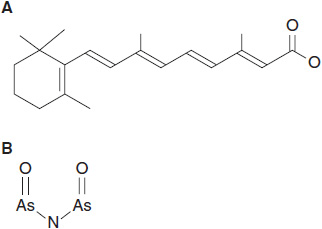
FIGURE 9-1 Differentiating agents useful in APL therapy. (A) All-trans retinoic acid (ATRA) and (B) arsenic trioxide.
ATRA
Retinoids (vitamin A and its derivatives) induce differentiation of malignant cells in cell culture systems. Early work showed that retinoids caused promyelocytic leukemia (APL) (HL-60) cells to undergo maturation into granulocytes at drug concentrations easily achievable in humans. In normal cells, RAR-alpha forms homodimers as well as heterodimers with retinoid X receptor, and the dimer in turn complexes with the PML transcription factor. The RAR-alpha homodimer in complex with PML in turn binds ATRA, leading to chromatin modification and transcription of genes that induce differentiation (2). In APL cells, the normal pathway for vitamin A action is disrupted by a translocation fusing portions of the RAR-alpha gene on chromosome 15 and the PML gene on chromosome 17. The fusion protein acts as a repressor of differentiation and, through the FOS gene, promotes proliferation. The RAR-alpha/PML fusion protein has low affinity for ATRA and requires pharmacologic concentrations of retinoid to activate differentiation in APL cells. High concentrations of ATRA lead to multiple effects on leukemic cell differentiation: degradation of the fusion protein, chromatin modification, and upregulation of transcription factors (CEBP beta, OCT-1, and most importantly, PU1) required for myeloid differentiation (2). Resistance to ATRA arises through mutation of the fusion protein to decrease its binding affinity, and possibly by induction of the ATRA metabolizing p450 isoenzyme (CYP26A1) in the liver or in APL cells after prolonged exposure to the drug (3).
 CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
ATRA induces complete remission in more than 90% of patients with APL, alone or in combination with an anthracycline (1). The initial clinical experience with ATRA revealed clear evidence of leukemic cell maturation in bone marrow and peripheral blood. Remission duration for single agent ATRA tends to be brief, and the recurrent tumor is usually refractory to a second cycle of treatment due to further mutation in the RAR-alpha gene. However, when ATRA is used in combination with anthracyclines as primary therapy, the regimen is curative for 70% or more of patients with this disease. It is effective in all patients with the PML/RAR-alpha translocation, including those with a masked translocation, only detectable by PCR. The “long” form of the translocation is more sensitive to APL and has a greater disease-free survival after remission than does the “short” form (4).
ATRA is given in daily intravenous doses of 45 mg/m2/day until remission is achieved. It is now used as the initial therapy in APL in combination with an anthracycline. ATRA has the additional advantage of quickly aborting the life-threatening coagulopathy associated with this disease. In patients with significant comorbidities or in elderly patients, it efficiently induces remission when used with arsenic trioxide, without a cytotoxic agent. It may also be effective in maintenance therapy, but its continuous use leads to cutaneous toxicity and elevated plasma triglycerides. Therefore, intermittent schedules of maintenance ATRA, given with methotrexate and 6-mercaptopurine, may be better tolerated and equally efficacious. ATRA concentrations in plasma reach 400 ng/ml, and ATRA is rapidly eliminated by p450-mediated metabolism. The half-life in plasma is less than 1 h, and the rate of clearance increases markedly with repeated doses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree