Fig. 6.1
Coronal contrast-enhanced computed tomography (CT) image showing (a) multiple nodules with a diameter > 5 mm diffusely involving the tunica serosa over bowel loops and paracolic gutters (arrows), (b) as documented during surgical intervention (arrow)
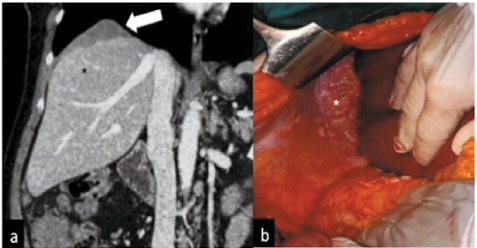
Fig. 6.2
Coronal computed tomography (CT) image showing (a) irregular soft tissue (arrow) of inconstant extension formed by the confluence of multiple nodular implants in the right subdiaphragmatic space scalloping the liver surface and presenting a lower density than the parenchyma (black asterisk) on contrast-enhanced scan. b During surgical intervention, the pathological subdiaphragmatic tissue was confirmed (white asterisk)
6.2.3 Disadvantages of Computed Tomography
In the literature, overall sensitivity and specificity of contrast-enhanced (CE) MDCT in identifying peritoneal deposits vary substantially, ranging from 25 % to 93 % [6, 16] and from 78 % to 98 %, respectively [6, 17]. The reason for this wide variability is multifactorial, including differences in tumor characteristics (size or density), patient features (CT assessment of peritoneal metastases is more difficult in very thin patients), radiologist expertise, diagnostic criteria used in CT interpretation, and CT techniques. Coakley et al. reported an overall sensitivity of 85–93 % for implants > 10 mm that decreased to 25–50 % for implants < 10 mm [16]. However, there was no information about the type of spiral scanner used and section collimation was 5 mm, 7–8 mm, or 10 mm [16]. Even using an advanced CT technique, for small peritoneal nodules, detection rate for peritoneal metastases is not greatly improved. In particular, when malignant peritoneal deposits have a low volume (< 5 mm), reported CT sensitivity tends to decrease [6, 18]. Marin et al., using a 64-row CT scanner with a more accurate technique (effective section thickness and reconstruction interval 3 mm), assessed preoperatively PC in 18 patients with different neoplasm types. They found a mean sensitivity of 89 % for lesions ≥ 5 mm but a sensitivity of only 43 % for lesions < 5 mm in diameter [7]. Identifying neoplastic implants in challenging anatomic sites represents another disadvantage of CT. In the literature, the reported per-site sensitivity for the exact location of peritoneal implants is only 25–37 % [14].
6.3 Magnetic Resonance Imaging
6.3.1 Imaging Technique
MRI for peritoneal malignancies is performed using a high-field system (1.5–3.0 Tesla). Larger phased-array surface coils or the use of two-array coils combined provides simultaneous coverage of the abdomen and pelvis [2]. The protocol includes patient fasting for 8 h, oral administration of water (500 ml), and intramuscular administration of hyoscine butylbromide (Buscopan) before pelvic scans. Abdominal imaging comprises T2-weighted fast spin-echo (SE) axial and coronal imaging and for the pelvis comprises high-resolution T2-weighted fast SE sequences in axial, coronal, and sagittal planes. Gadolinium administration IV is not mandatory for implant detection, but if it is administrated, postenhanced acquisitions should be performed with a delay of ~ 5 min. DWI should be performed for both abdomen and pelvis, acquiring axial scans using breathhold, single-shot SE planar sequences. Sensitivity of DWI sequences for hypercellular tissue can be increased by increasing the b value; acquisition using a b value of 0 s/mm2, 800 s/mm2, and 1,000 s/mm2 is usually satisfactory. All axial DWI images can also be depicted with black-and-white reverse-contrast display, and apparent diffusion coefficient (ADC) maps are usually available to provide quantitative assessment [2].
6.3.2 Advantages of Conventional Magnetic Resonance with Diffusion-weighted Imaging
Over the last decade, the role of MRI in peritoneal malignancy has significantly increased, primarily due to technical improvements and wider availability. Using conventional sequences with DWI is comparable with MDCT for detecting peritoneal deposits (> 1 cm) and in certain cases surpasses that of CT sensitivity and specificity [19–21]. The reported sensitivity and specificity of conventional MRI with DWI is 90 % and 95.5 %, respectively [20]. Combining traditional MR sequences and DWI increases accuracy for peritoneal implants measuring < 10 mm and for peritoneal implants located in sites difficult to evaluate on CT. In particular, conventional MRI with DWI seems to improve detection of malignant deposits over subdiaphragmatic spaces, hepatic hilum, Treitz ligament, smallbowel wall, and mesenteric root [22–25], which are crucial sites to definitely or temporarily exclude patient candidacy for surgery (Fig. 6.3). Moreover, using high-resolution MR sequences to study the pelvis is the better imaging modality for predicting primary or metastatic gynecological malignancies due to its superior spatial and contrast resolution [21, 22].
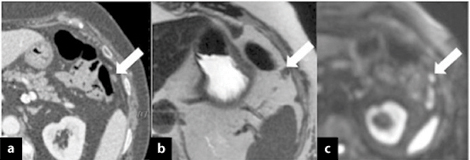

Fig. 6.3
Axial computed tomography (CT) image showing (a) a solid nodule < 5 mm near the splenic flexure (arrow) suspected to be fibrotic tissue in a patient with sigmoid cancer (pT3N+) treated with surgical intervention and adjuvant chemotherapy 6 months earlier. b Axial T2- weighted magnetic resonance image (MRI) better depicts the small, solid nodule (arrow) due to the high-contrast resolution. c At diffusion-weighted imaging (DWI), the nodule shows hypercellularity and is strongly suspected to be a peritoneal metastasis
DWI is a functional magnetic resonance imaging (fMRI) technique in which the signal comes from the restricted water mobility typically present within hypercellular tumors. This high signal intensity of neoplastic nodules increases the contrast between the site of neoplastic disease and surrounding tissue [20]. Increased contrast between malignant tissue and around normal tissue makes it easier to detect smaller implants, which appear as hyperintense spots surrounded by hypointense normal tissue, due to signal suppression of surrounding ascites, bowel contents, and fat, with an increased contrast-to-noise ratio (CNR).
DWI also allows quantitative assessment by calculating the ADC of each voxel, which occurs automatically on current MRI systems by assuming a monoexponential model of signal decay between two or more b values. Therefore, once the ADC value is determined, the MR system can displayed a parametric map that essentially reflects differences in tissue diffusivity at different b values [22]. Integration between conventional MRI, dimensional criteria, and DWI seems also to improve the ability to assess metastatic lymph nodes by 17–21 %. Many studies found that inflammatory lymph nodes present higher value on ADC maps than metastatic nodes [26].
In the early posttreatment period, conventional MRI and DWI can be helpful in distinguishing between inflammatory tissues and residual disease. In fact, highintensity signal on T2-weighted sequences, unrestricted signal on DWI, and high value on ADC mapping is frequently related to postsurgical edema and inflammation, whereas restriction on DWI and low ADC value is suggestive of active tumor cells [26].
The correct way to quantify and assess tumor response to treatment is evaluation of tumor dimensional changes on CT or MRI and laboratory tests for tumor markers. Furthermore, a high value on ADC mapping is related to a positive treatment response. That is because chemotherapy causes necrosis inside the tumor, resulting in increased diffusion of water molecules and consequently increased ADC value.
6.3.3 Disadvantages of Conventional Magnetic Resonance with Diffusion-weighted Imaging
Disadvantages of MRI are represented by high cost, long acquisition time, and motion and susceptibility artifacts. Disadvantages of DWI are mainly related to low spatial resolution and some possible pitfalls. Sometimes, tissues presenting long T2 relaxation time, such as cysts or postsurgery edema that may appear hyperintense on high b values, is a phenomenon called T2 shine through. That artifact can cause misinterpretation of radiological images, inducing false positive findings; ADC mapping can rectify this problem. On the other hand, a low ADC value suggests real restriction of water diffusion [22], which indicates neoplastic disease.
As mentioned earlier, the increased cell density on DWI permits recognition of neoplastic tissues due to restriction of water movement represented by high signal intensity on high b value and low signal intensity on ADC mapping. However, some types of tumor, such mucinous tumors and well-differentiated adenocarcinomas, have a lower cell density and therefore may show low signal intensity on high b values [22]. Moreover, some densely cellular normal tissue, such as bowel mucosa, endometrium, and normal lymph nodes, present restricted diffusion appearing hyperintense in DWI with low ADC values. In posttreatment follow-up, anatomical modification in the postoperative abdomen and the small size of recurrent tumors decrease diagnostic accuracy due to formation of fibrotic tissue that can present restricted diffusion and low ADC values [22]. Necrosis and abscesses also have high values on DWI sequences with high b values but generally present higher values on the ADC map [22] and different enhancement after contrast administration. Therefore, interpretation of DW images needs to be performed in conjunction with conventional MRI.
6.4 Positron Emission Tomography/Computed Tomography
6.4.1 Imaging Technique
In clinical practice, PET with [18F]-fluorodeoxyglucose (FDG) PET has significantly improved in diagnostic accuracy and now exerts a major impact on patient management, in particular for diagnosis, staging, therapy monitoring, and restaging recurrent disease. [18F]-FDG-PET is based on identifying the increase in glycolytic activity in malignant cells, which preferably concentrate glucose due to increased membrane transporters of glucose (GLUT-1). Imaging systems that combine [18F]-FDG-PET and CT allow simultaneous evaluation. Anatomical and functional total-body [18F]-FDG-PET/CT imaging is routinely performed using the Hamacher method of injecting patients with 5 MBq/kg of [18F]-FDG hydrated with 500 ml of saline solution. PET/CT acquisition in 2D mode begins 60 min after IV administration. The axis of both systems is mechanically aligned to move the patient from the CT into the PET gantry by moving the examination table by 60 cm. Finally, PET and CT images are coregistered on hardware. Patients are usually scanned in the supine position, starting from the head and moving to just above the first scanning position on the CT scanner. Before scanning the patient, a scout view is obtained to define axial imaging range. Acquisition parameters are 120 kV, 80 mA, tube-rotation time 0.8 s, pitch 1.5).
PET/CT is performed after CT, covering the same field of view (FOV) and with acquisition time ~ 4 min per table position. Axial and other MPR images can be used to detect peritoneal disease and check the common peritoneal site of pathological involvement.
6.4.2 Advantages of Positron Emission Tomography/Computed Tomography
Combining morphological and functional imaging has clear advantages in oncological imaging, particularly for evaluating peritoneal malignancies. The role of PET in diagnosing malignant neoplastic disease in the ovary remains controversial. A recent study demonstrates good diagnostic accuracy in differentiating malignant and benign ovarian tumors; [18F]-FDG-PET shows 87–100 % sensitivity, 74–100 % specificity, and 92–97 % accuracy [27]. Detecting ovarian cancer (OC) depends on lesion the size. This may be due to resolution limitations with PET despite the availability of integrated PET/CT systems, for which diagnosing microscopic lesions remains barely feasible.
The key role of [18F]-FDG-PET in PC evaluation is the presurgical evaluation of abdominal and retroperitoneal lymph node and distant metastases (N/M), in which functional imaging may have a higher morphology. Kitajima et al. reported that in detecting pelvic metastases and para-aortic lymph nodes, CT sensitivity, specificity, and accuracy were, respectively, 37.5 %, 100 % and 86.5 %, whereas those of PET/CE-CT were 81.3 %, 96.6 %, and 93.2 % [27, 28].
Morphological imaging, however, is limited in detecting early response to therapy because anatomical changes are usually visible only 2–3 months after therapy, whereas metabolic changes appear after two to three sessions of chemotherapy. Functional imaging using PET/CT is somewhat crucial in identifying nonresponsive patients, thus improving patient management, avoiding the use of ineffective therapies, helping avoid adverse effects, and reducing delay before administering a more effective treatment, thus decreasing costs.
Nishiyama et al. shown that reducing the standard uptake value (SUV) after the first cycle of chemotherapy can predict response to chemotherapy or chemoradiotherapy in patients with gynecological cancers (uterine, n = 13; ovarian, n = 8) [29]: on the basis of histopathological analysis of surgical samples, 10 patients were responsive and 11 nonresponsive. Considering a cutoff SUV of 3.8 to differentiate between responsive and nonresponsive patients, [18F]-FDGPET showed a sensitivity of 90 %, a specificity of 63.6 %, and an accuracy of 76.2 %; considering SUV variation of 65 %, [18F]-FDG-PET showed a sensitivity of 90 %, a specificity of 81.8 %, and an accuracy of 85.7 %.
In the course of restaging after initial debulking surgery and chemotherapy in first-line treatment, it is important to assess the possible presence of residual or recurrence of disease during follow-up. Approximately 20–30 % of patients with early-stage disease and 50–75 % with advanced disease who obtain complete response after first-line therapy may experience subsequent relapse. The clinical follow-up generally includes serum CA 125, physical examination, and conventional imaging tests.
CT and MRI are the imaging methods most commonly used in patients with suspected recurrence of OC; however, they have limitations in distinguishing residual tumor from necrosis or fibrosis and in characterizing distant lymph node, bone, and muscle metastases. [18F]-FDG-PET compared with physical examination and CT scan shows a sensitivity of 73–100 %, a specificity of 71–100 %, and accuracy of 83–100 % [30], primarily due to the high-contrast resolution (Fig. 6.4). However, compared with [18F]-FDG-PET plus histopathology obtained during a second-look laparotomy evaluation, the diagnostic accuracy of [18F]-FDG-PET alone tends to be lower, with a reported sensitivity, specificity, and accuracy of 53–83 %, 40–86 %, and 63–82 %, respectively [30]. These discrepancies are attributable to the fact that small lesions may not be seen due to the lower resolution of [18F]-FDG-PET. Therefore, [18F]-FDG-PET is a noninvasive imaging methodology that is more accurate for restaging, especially for assessing peritoneal spread and lymph node, bone, and muscle metastases because it allows imaging of the entire body in a single examination.
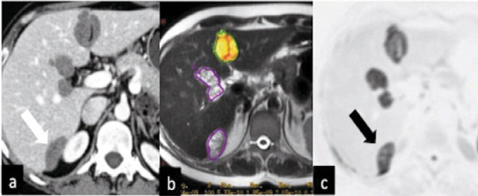

Fig. 6.4
Peritoneal cystic implants (arrows) above the liver surface (at the hilum, near the falciform ligament, and within Morison’s pouch) on (a) axial computed tomography (CT), (b) axial magnetic resonance imaging (MRI), and (c) on positron emission tomography (PET/CT) in a patient with metastatic ovarian cancer. PET/CT shows the highest contrast resolution
6.4.3 Disadvantages of Positron Emission Tomography/Computed Tomography
The disadvantages of PET/CT imaging are mainly related to high costs and poor availability of the equipment around the world, which often prevents its routine use. Another important drawback of PET/CT is its lower spatial resolution and lower sensitivity for lesions < 1 cm, resulting in reduced sensitivity in detecting small implants, particularly during the first staging.
6.5 Preoperative Staging of Peritoneal Disease
The task of preoperative imaging is to determine accurate extension of peritoneal disease, stratifying patients who are good surgical candidates from those who may be candidates for NACT in an attempt to reduce tumor burden. The PCI, introduced by Jacquet and Sugarbaker [13], is considered the most accurate system for staging PC from different primary tumor types based on quantification and distribution of peritoneal implants found at laparotomy. According to this method, the abdominopelvic area is subdivided into 13 sections (nine areas plus relating to the small bowel) (Fig. 6.5). PC lesion size is rated on a fourpoint scale ranging from 0 to 3 points, with a possible maximum score of 39: LS 0, no tumor detected; LS 1, tumor up to 0.5 cm maximum diameter, LS 2, tumor > 0.5 cm up to 5 cm in maximum diameter; LS 3, tumor or confluent lesions > 5 cm in maximum diameter [31].
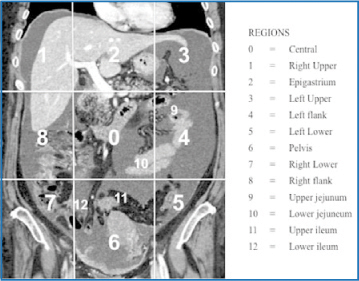

Fig. 6.5
Preoperative staging of peritoneal carcinomatosis (PC). The abdomiopelvis is virtually subdivided into 13 areas: nine areas plus four relating to the small bowel
The PCI score also represents a widely validated and precise quantitative prognostic indicator [2, 3], predicting whether complete resection of peritoneal implants can be achieved after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CRS plus HIPEC). Assessing potentially nonresectable or nonoptimally cytoreducible disease that can contraindicate CRS plus HIPEC comprises the following diagnostic criteria [32]:
Extensive involvement of the small bowel or mesenteric root
Involved lymph node superior to the celiac axis
Pleural infiltration
Pelvic sidewall invasion
Bladder trigone involvement
Hepatic parenchymal metastases or implants near the right hepatic vein or the porta hepatis
6.5.1 Preoperative Assessment of Peritoneal Cancer Index: Comparison between Imaging Modalities
6.5.1.1 Multidetector Computed Tomography
MDCT, using an adequate diagnostic technique, has a high mean sensitivity of 89 % for implants measuring ≥ 5 mm but a sensitivity of 43 % for implants with a diameter < 5 mm [7].
Main advantages:
Wide availability
Short execution time
Lower cost
Multiplanar imaging
Thin section (1–3 mm)
High spatial resolution
Few or absent artifacts
Main disadvantages:
Low contrast resolution
Moderate sensitivity for implants < 5 mm
Diagnostic accuracy strictly depending on CT technique and radiologist experience
MDCT remains the modality of choice for primary staging, especially in patients with poor compliance for diagnostic examinations, providing a great deal of information about a large volume of tissue in just a few minutes and permitting assessment of metastatic extraperitoneal disease.
6.5.1.2 Magnetic Resonance Imaging
MRI offers excellent soft-tissue contrast to depict small-volume peritoneal tumors using both conventional and DWI sequences. The reported overall sensitivity of conventional MRI with DWI for PC assessment is 90 % [21], and sensitivity for lesions < 10 mm remains satisfactory, corresponding to 85 %, which is higher than that of CT, which ranges from 25 % to 50 %.
Main Advantages:
High-contrast resolution
High sensitivity also for implants < 10 mm
Free from radiation exposure
Multiplanar imaging
Higher spatial resolution in the studies of pelvic malignancies
DWI with morphological unenhanced sequences offers an accurate alterative method in case of reported allergy or renal failure, which contraindicate enhanced MDCT
Main Disadvantages
Every contraindication to MRI must be considered
Lack of relative availability
Not recommended in patients with poor compliance
Higher costs in some centers
Imaging artifacts
Longer acquisition time
Metastatic extra-abdominal disease is not evaluated
Difficulties in differentiating between postsurgery edema, cysts, and other benign findings
Diagnostic accuracy strictly depends on MRI technique and radiologist experience
MRI with conventional and DWI should be always considered the modality of choice for primary staging of abdominal disease, especially in young and tolerant patients. In those cases, additional CT to study the thorax should be also considered.
MRI with conventional and DWI can be performed after MDCT in cases in which CT is executed with a suboptimal technique or if equivocal CT findings requiring a superior diagnostic method to achieve detailed surgical planning or determine whether the patient needs neoadjuvant chemotherapy.
6.5.1.3 [18F]-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography
[18F]-FDG-PET/CT provides metabolic information enabling the identification of malignant lesions with high-contrast resolution due to their augmented glucose consumption. Klumpp et al. reported sensitivity and specificity values for PET/CT of 92–93 % and 94–96 %, respectively, which is higher than MRI (sensitivity 87 %, specificity 86–92 %) [37]. In a meta-analysis, Chang et al. reported an overall pooled estimated sensitivity and specificity of FDG-PET or PET/CT scans in PC detection of 72.4 % and 96.7 %, respectively [38].
High sensitivity and specificity
Higher contrast resolution than conventional MDCT
Multiplanar imaging
Few or absent artifacts
Better interobserver agreement in comparison with MDCT and MRI, as well as better correlation
Main Disadvantages:
Poor availability
High cost
Lower spatial resolution
Long execution time
6.5.2 Personal Experience
In June 2008, our team created a tight connection with a group of surgeons from the Department of Surgery, “Sapienza” University, Rome, who seldom use HIPEC, in an attempt to evaluate MDCT accuracy in the preoperative definition of the PCI in patients with advanced OC, colonic, and gastric cancer who underwent peritonectomy (PRT) plus HIPEC. In our series, we evaluated up 120 patients affected by ovarian, gastric, and colonic carcinomatosis, attaining very good sensitivity (up to 90 %) in evaluation of the 13 abdominopelvic areas, with overall sensitivity of ~ 85 % and acceptable overall specificity for all regions except for mesenteric root, pelvic sidewall invasion, and Treitz ligament region, in which specificity values varied between reader experience from 40–65 %. Due to the poor results in the latter instances, in 2012, we began using DW-MRI for patients in whom results were unclear. In some patients, we obtained better specificity in the mesenteric root, porta hepatis, and pelvic sidewall in comparison with MDCT. Of the 13 abdominopelvic areas, the best results were obtained in the splenic hilum (region 3), with sensitivity 91 % and specificity 90 %, and overall positive (PPV) and negative (NPV) predictive values of 95 % and 88 %, respectively. This was also the case for both hemidiaphragms, with 94 % and 95 % sensitivity and specificity and PPV and NPV 90 % and 90 %, respectively
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree