Diagnosis of Hypothyroidism
Paul W. Ladenson
Since the syndrome of myxedema was described more than a century ago, the criteria for diagnosis of hypothyroidism have evolved from clinical observation to bioassays, increasingly specific measurements of thyroid hormones in serum, and quantitation of endogenous thyrotropin (thyroid-stimulating hormone, TSH) production. An accurate diagnosis of hypothyroidism requires awareness of the clinical features that define a patient’s risk for thyroid hormone deficiency and proper use of the two tests usually required to confirm the disorder: Serum TSH and free thyroxine (T4) assays (1). These sensitive and specific measures of thyroid function have largely resolved the inaccuracy associated with the clinical diagnosis of hypothyroidism. However, they also have introduced new diagnostic challenges. First, we now appreciate that there are many patients with few clinical manifestations of hypothyroidism and mild thyroid hormone deficiency that is revealed only by serum TSH measurements—a disorder defined as mild or subclinical hypothyroidism (2). Second, thyroid test abnormalities are common in patients with nonthyroidal illness (3). Consequently, clinicians must still integrate clinical observations with laboratory test results to properly diagnose and manage patients with hypothyroidism.
Clinical Diagnosis
Three types of clinical evidence suggest hypothyroidism: Symptoms and signs consistent with thyroid hormone deficiency; evidence of diseases, previous treatment, or exposure known to cause thyroid or pituitary failure; and the presence of disorders associated with increased risk of chronic autoimmune thyroiditis.
Clinical Manifestations
Thyroid hormone deficiency may present as an obvious clinical syndrome (e.g., cretinism or myxedema). Clinical manifestations are also often present in patients with mild to moderate hypothyroidism. In one large population survey, individuals with a greater number of typical symptoms, particularly if they were new complaints, were more likely to have thyroid function test results that confirm the diagnosis (4). However, symptoms were present in only 30% of biochemically hypothyroid patients, whereas 17% of euthyroid people had the same complaints. As a result, the positive predictive value of individual symptoms was only 8% to 12%. Consequently, although symptom scoring scales with significant predictive power have been described (5), they remain too insensitive and nonspecific to diagnose or exclude hypothyroidism definitively (6).
Even in patients with overt biochemical hypothyroidism, symptoms and signs may be minimal or absent (7). Several factors account for the subtlety with which hypothyroidism can present: Its insidious onset, the presence of mild thyroid hormone deficiency, and the passivity and lack of insight that accompanies hypothyroidism in some patients. Furthermore, many of the symptoms of hypothyroidism are nonspecific (e.g., fatigue and constipation), as are several of the physical findings (e.g., dry skin and weight gain). Among patients with symptoms potentially attributable to hypothyroidism in a primary care setting, the diagnosis is established in only 1% to 4% (8,9). Moreover, hypothyroidism can have age- and sex-specific presentations (e.g., impaired growth in children, menorrhagia in menstruating women, and impaired cognition in older adults). Furthermore, older individuals tend to have fewer hypothyroid symptoms than do younger individuals (10).
Predisposition to Hypothyroidism
Hypothyroidism should be suspected when there is evidence of an underlying thyroid, pituitary, or hypothalamic disorder known to cause thyroid failure, or when some previous treatment has destroyed thyroid, pituitary, or hypothalamic tissue. For example, the presence of a diffuse goiter, a common manifestation of chronic autoimmune thyroiditis, should prompt laboratory evaluation for hypothyroidism (see Chapter 35). A history of previous thyroid surgery or radioactive iodine therapy likewise suggests possible primary hypothyroidism, which can occur either soon or many years after these treatments. This is particularly true in patients with Graves’ disease, which may eventually cause hypothyroidism even without destructive therapy (11). Radiation therapy to the cervical region for lymphoma (12) and head and neck cancers (13) also frequently causes hypothyroidism (see Chapter 37).
Clinical evidence of hypothalamic or pituitary disease should raise suspicion of central hypothyroidism, also known as secondary or thyrotropic hypothyroidism (see Chapter 38) (9). Hypothalamic disorders that can cause thyrotropin-releasing hormone (TRH) deficiency include tumors and granulomatous diseases. The pituitary’s thyrotropic cells can be affected by endocrine and other tumors, treatment for them, inflammation, or hemorrhage. Therefore, other evidence of hypopituitarism, such as growth failure, hypogonadism, adrenal insufficiency, or diabetes insipidus; an expanding sellar mass lesion (e.g., headache, bitemporal hemianopsia), or a pituitary tumor secreting hormones other than TSH should prompt evaluation for hypothyroidism.
Risk factors for hypothyroidism include a variety of drugs, of which lithium carbonate and amiodarone are the most commonly used (14) (see Chapter 11B). Iodine in pharmacologic quantities, such as that occurs with amiodarone therapy (15), can cause hypothyroidism in patients with underlying autoimmune thyroiditis by further interfering with thyroid hormone
synthesis and release (see Chapter 11E). The anti-seizure medications phenytoin and carbamazepine, and the antituberculous agents rifampin and rifabutin, which increase the clearance of T4, can cause hypothyroidism in patients with limited thyroid reserve (16) (see Chapter 11B). The tyrosine kinase inhibitor sunitinib can also cause hypothyroidism by accelerating thyroid hormone disposal (17). Patients with mycosis fungoides who are treated with the selective retinoid X receptor ligand bexarotene can develop central hypothyroidism due to the drug’s suppression of TSH production (18).
synthesis and release (see Chapter 11E). The anti-seizure medications phenytoin and carbamazepine, and the antituberculous agents rifampin and rifabutin, which increase the clearance of T4, can cause hypothyroidism in patients with limited thyroid reserve (16) (see Chapter 11B). The tyrosine kinase inhibitor sunitinib can also cause hypothyroidism by accelerating thyroid hormone disposal (17). Patients with mycosis fungoides who are treated with the selective retinoid X receptor ligand bexarotene can develop central hypothyroidism due to the drug’s suppression of TSH production (18).
Chronic Autoimmune Thyroiditis and Hypothyroidism
The patient’s personal and family histories and the presence of a goiter on physical examination may provide clues to the presence of chronic autoimmune thyroiditis and therefore the presence of hypothyroidism. Treatment with interferon-α has been associated with a high incidence of autoimmune thyroiditis and hypothyroidism that can be reversible after use of the agent is discontinued (19). Because of the genetic predisposition to chronic autoimmune thyroiditis, patients with affected family members are at increased risk for having hypothyroidism; postpartum women are at particular risk. Certain other endocrine deficiencies believed to have an autoimmune pathogenesis are also associated with chronic autoimmune thyroiditis, including idiopathic adrenal insufficiency, autoimmune diabetes mellitus, hypoparathyroidism, and primary ovarian failure. Patients with several other autoimmune disorders, including vitiligo, atrophic gastritis, pernicious anemia, systemic sclerosis, Sjögren’s syndrome, and celiac disease, are also at increased risk of autoimmune thyroiditis.
Laboratory Testing
Several abnormalities in routine laboratory test results may suggest hypothyroidism, but are not specific for it. They include hyperlipidemias [i.e., elevated total and low-density lipoprotein (LDL) cholesterol, triglycerides, and lipoprotein(a) levels], hyperprolactinemia, anemia, hyponatremia, hyperhomocysteinemia, and elevated levels of the skeletal muscle-associated isoenzymes creatine phosphokinase and lactic dehydrogenase. Other abnormalities that may indicate the presence of hypothyroidism are X-ray or echocardiographic evidence of pericardial effusion or impaired cardiac diastolic or systolic functions. Diffuse flat T waves and low voltage can also be present in patients with overt hypothyroidism.
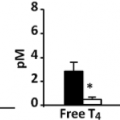
Failure of thyroid hormone production causes a decline in the serum T4 concentration. Serum triiodothyronine (T3) concentrations also decline, but are not frankly low until hypothyroidism is severe due to compensatory increases in thyroidal T3-secretion and hepatic T4-to-T3 conversion. Most of the patients with overt hypothyroidism have primary thyroid hypofunction, which results in low serum T4 and high serum TSH levels. The serum TSH concentration can be elevated despite a normal free serum T4 concentration when hypothyroidism is mild (subclinical hypothyroidism), as the T4 setpoint for an individual is considerably narrower than the entire reference range (20). Patients with central hypothyroidism, who are much less common, typically have low or low-normal serum T4 concentration with a low or low-normal serum TSH. Some individuals with central hypothyroidism can actually have a modestly elevated serum TSH concentration (e.g., 5 to 7 mU/L) due to the secretion of TSH that is immunoreactive but less biologically active (21). It is important to remember that other conditions can generate potential confusion in diagnosis of (see next sections).
Serum Thyroxine Determinations
The serum total T4 concentration can be measured accurately by competitive protein-binding assays that use either anti-T4 antibodies or serum thyroid hormone–binding proteins (THBP) (see Chapter 13A). Since most (99.97%) of the T4 in serum is bound to thyroxine-binding globulin (TBG), transthyretin (TTR, or thyroxine-binding prealbumin), and albumin, low concentrations of one or more of these serum proteins or drug-induced inhibition of T4 binding to them results in a low serum total T4 concentration (hypothyroxinemia) that can be misconstrued as indicating hypothyroidism (Table 45.1). Conversely, increased serum protein binding of T4 may mask hypothyroidism.
Table 45.1 Abnormalities in Serum Thyroid Hormone Binding that can Mimic or Mask Hypothyroidism | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Measurement of serum free T4 resolves most of these problems. Although equilibrium dialysis and ultrafiltration are the most accurate methods for determining serum free T4, serum free T4 radioimmunoassays and the serum free T4 index are adequate for this purpose in most circumstances, and they are simpler, quicker, and less costly. Almost all serum free T4 assays have excellent sensitivity for detection of hypothyroidism in symptomatic patients. The serum free T4 index is the product of the serum total T4 and the thyroid hormone binding ratio (THBR), also known as the T3 uptake or the T4 uptake (22) (see Chapter 13A). Serum free T4 assay techniques usually
yield comparable values. The THBR value, however, can sometimes provide additional useful information in patients with non-thyroidal illness, in whom the values are often normal or high despite low calculated serum free T4 index values and free T4 concentrations; in contrast, THBR values are low in most patients with hypothyroidism (23).
yield comparable values. The THBR value, however, can sometimes provide additional useful information in patients with non-thyroidal illness, in whom the values are often normal or high despite low calculated serum free T4 index values and free T4 concentrations; in contrast, THBR values are low in most patients with hypothyroidism (23).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree