Fig. 4.1
General classification of hip fractures
The overall incidence of femoral neck fractures in the USA is between 0.4 and 1.0 % [15, 16], and the estimated cost of treatment of femoral neck fracture alone has been estimated at $19,000–$23,000 for 1 year of treatment [17–19]. With the demographical aging of the population, the number of fractures is expected to increase, and it is reasonable to expect that cost of treatment will increase as well. Over 12 years in a British hospital, there has been a 10 % increase in admissions from hip fracture from 2000 to 2012, and it has been considered to be a nonlinear increase over time [20].
Type I DM has a definite effect on the incidence of hip fractures with reported relative risks of between 1.7 and 12 times higher than controls, with a suggested average of sixfold increase [21–24]. In addition, type I DM patients have hip fractures at a younger age on average, with a mean of 43 for women and 41 for men in one study. Almost 7 % of people with type I DM can be expected to have sustained a hip fracture by age 65 [7]. The mechanisms that cause these fractures in type I DM are unclear, but three main reasons have been speculated.
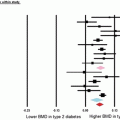
First, bone mineral density (BMD) in patients with DM type I is decreased as compared with controls [25]. One large meta-analysis estimates that the hip Z-scores are −0.37 from expected, and this decrease in BMD suggests a 1.42 increase in risk of hip fracture. However the relative risk is somewhere about sixfold over nondiabetic people. This discrepancy has suggested that other pathological mechanisms should be explored to help explain the increased risk of hip fracture in type I DM [26].
Second, type I DM is commonly associated with decreased proprioception, neuropathic changes, and retinopathy with increasing duration and severity of disease process. Each of these comorbidities can potentially contribute to falls, increasing the chances of hip fracture over time [7, 27, 28]. In addition, these disease processes could serve as a marker of severity, and may also have some contribution to pathological bone homeostasis. Supporting these hypotheses, the presence of ophthalmic, neurological, and cardiovascular complications increase the risks of hip fracture by 20, 41, and 29 times expected general population values. Furthermore, as compared to others with type I DM without complications, the risks are 1.3, 2.0, and 1.7, respectively [7]. Finally, hypoglycemia has been suggested as a possible cause, as type I DM is treated with various types of insulin, but all can lead to hypoglycemia [27, 29].
Compared to patients with type I DM, the increased incidence of hip fracture in patients with type II DM is less certain, but is likely. Conflicting evidence has suggested an increased risk, no change in risk, and even a decreased risk of fracture [10, 21, 28, 30–35]. However, the reports on the largest enrollments generally put the relative risk between 1.2 and 2.2, and most recent studies suggest an increased relative risk [14]. It is somewhat confusing that patients with type II DM also have increased BMD on average; however, some propose that the BMD difference is related to the confounding variable of obesity rather than solely a direct effect [25, 26, 30, 33, 34]. The duration of diabetes has also been suggested as a risk factor [31], but age is a significant confounding variable to be considered.
The medical treatment regimens for patients with diabetes have also been suggested as a possible risk factor, given a two times higher risk of hip fracture in patients with a HbA1c of <6.0 vs. those with HbA1c levels greater than 8 %. The tighter control of diabetes could result in more episodes of hypoglycemia, which in turn can potentially lead to more falls [27]. To support this hypothesis, the use of metformin and acarbose has been associated with a decreased risk of hip fractures in type II DM as compared to patients treated with insulin or sulfonylureas. Metformin and acarbose have a much smaller risk of hypoglycemia, and hypothetically may lead to less falls. Thiazolidinediones have also been implicated in higher rates of hip fracture, but not universally [21, 27, 36]. However, hypoglycemia as a potential reason for falls is controversial, as some research suggests that intensive glucose control does not increase the propensity for falls [37].
Treatment for hip fractures is almost exclusively operative, as nonsurgical treatments result in high rates of nonunion, pain, decubitus ulcers, pneumonia, deep venous thrombosis, and pulmonary embolus [38, 39]. For femoral neck fractures, treatment options are open reduction and internal fixation (ORIF), percutaneous screw fixation, hemiarthroplasty, and total hip arthroplasty (THA). Although somewhat controversial, there has been a trend towards total hip replacement in patients with femoral neck fracture despite many having intact cartilage. This trend is because the long-term outcomes after THA are superior to other methods of treatment, in spite of having some increase in early complications [40–43]. With ORIF, there are late complications of fracture nonunion and avascular necrosis of the femoral head. These complications are thought to be related to damage of the femoral head blood supply and the resulting hemarthrosis after displaced femoral neck fractures [44, 45]. In hip arthroplasty, the proximal segment of the femur is replaced and the problems of nonunion and avascular necrosis of the femoral head are no longer issues. For intertrochanteric and subtrochanteric hip fractures, the most accepted treatment is with ORIF, as the blood supply generally allows the fracture to heal, without significant risk of avascular necrosis [46].
Mortality rates after hip fracture at 1 year range from 18 to 33 % [3, 5, 8, 9, 47], and the rates remain higher than the general public for at least 10 years postoperatively [11, 12]. An analysis in the Danish registry noted that the 1 year mortality is nearly double that of those without femoral neck fracture, and that the excess mortality is approximately 1.8 % increased each year after. At 20 years follow-up, the survival is 57 % of what would be expected from the control group [11]. Patients with DM and hip fracture are at a higher risk of mortality than patients without DM, with 1-year rates as high as 32 % vs. 13 % of nondiabetic patients [3, 4]. In addition, DM is associated with an increased risk of cardiovascular, pressure ulcer, and infectious complications after hip fracture [4, 9, 48]. These complications of hip fracture treatment are thought to be the cause for increase in the risk of mortality, not just the presence of pre-existing diabetes [11].
Ankle Fracture
Fractures around the ankle are some of the most commonly seen fractures in the hospital setting and the incidence appears to be increasing, as a Finnish study has suggested a threefold increase in incidence by 2030 [49]. In diabetic patients, the concern is not necessarily an increased risk of fracture, but of treatment complications. Diabetic patients have significant increased risks of infection, malunion, neuropathic ankle, and amputation after ankle fractures, even those that are low energy injuries [50–58].
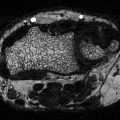
Ankle fractures consist of one or more fractures of the medial malleolus of the tibia, the lateral malleolus of the fibula (Fig. 4.2), and sometimes the posterior malleolus of the tibia [59]. A more significant, but related injury is a tibial plafond fracture. The plafond fracture is usually a higher energy injury with axial load, rather than a routine ankle fracture, which is a rotational injury [60]. The specific direction of forces that have created an ankle fracture classically determines the different fracture patterns [59]. Patient with complicated diabetes and no trauma may have Charcot or neuropathic arthropathy, and this type of presentation must be distinguished from those patients with a traumatic ankle fracture [55, 61, 62]. The ankle is an area of relative paucity of muscle tissue, and therefore the bony structures are covered with skin and subcutaneous tissue alone. Therefore, the associated soft tissue injury after ankle fracture is a more significant problem than with fractures in other locations in the body [63].
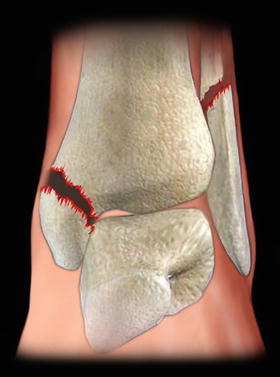

Fig. 4.2
Unstable ankle fracture
Treatment of ankle fractures can be either nonoperative or operative, depending on the stability of the ankle joint. The stability of ankle fractures is based on the integrity of the medial malleolus and the deltoid ligament. In an unstable ankle fracture, treatment is generally operative with anatomic reduction and fixation. In stable ankle fractures, the treatment is general nonoperative [64, 65].
Given the microvascular complications in diabetic patients, and with the relative paucity of soft tissue around the ankle, there is a great deal of concern with diabetic patients with unstable ankle fractures [66]. With nonoperative treatment, the complication rate in diabetic patients is high, with some studies stating an up to 83 % risk of complication in diabetics [57, 67]. Most of these complications relate to malunion and pressure sores from cast treatment, and the risks are understandably higher in those patients with neuropathy [50]. Although counter-intuitive, these studies are evidence that operative management of ankle fractures is indicated in those patients with more severe and complicated diabetes.
In diabetic patients treated with reduction and internal fixation, the complication rate is improved from nonoperative treatment, but still higher than controls at between 13 and 50 % [52, 56, 63, 68, 69]. This rate is compared to the reported risk of infections in controls of 1.4–7 % [55, 63, 68, 69]. The amputation rates are even more striking, with between 3.8 and 5 % of diabetics eventually ending with amputation after ankle fracture treatment, versus well under 1 % of nondiabetics [52, 56, 57, 67, 70, 71]. In open ankle fractures, the amputation rate in diabetic patients has been reported to be as high as 42 % [51], and is a strong predictor of increased complication rates overall [51, 70].
Peripheral neuropathy and microvascular complications are commonly cited as the underlying cause of these complications. First, the microvascular supply and the healing response are compromised in diabetic patients [66]. Second, peripheral neuropathy has loss of protective sensation in the foot and ankle, leading to pressure sores, noncompliance postoperatively, failure of fixation, and malunion of fractures. Indeed, patients with neuropathy have a reported 75 % complication rate after ORIF, and a 100 % complication rate treated with closed methods [50, 52, 55, 56, 67, 71]. Overall, those patients with peripheral neuropathy have a 15.5 odds ratio of a postoperative wound complication versus a control group [63].
Higher complication rates are seen in patients with HbA1c levels greater than 6.5 %, and optimization of blood glucose control is likely to be of benefit for overall outcomes [53, 72]. Emphasizing the importance of blood glucose control long-term, recent results suggest that the complication rate for those patients with uncomplicated diabetes is similar to nondiabetics [53]. More than 90 % of nondiabetic patients with ankle fractures will regain nearly all function after treatment, but only about 70 % of diabetic patients functionally recover, have longer hospital stays, and have higher overall hospital costs [54, 73]. There are two consensus recommendations for treatment of ankle fractures in diabetic patients. First, the ORIF fixation strength should be increased over what would be normally used for nondiabetic patients. Second, the postoperative nonweightbearing period should be lengthened over what would be used routinely, especially in those patients with complicated DM [58, 66].
Infection
Although there is a risk of infection associated with any orthopaedic surgery, patients with diabetes are at a higher risk of developing surgical site infections (SSIs) [51, 52, 57, 68, 74–77]. SSIs are associated with increased hospital stay, increased hospital cost, and reduced quality of life. A review of one hospital in the 1990s showed double mortality, double length of hospital stay, and double the cost expenditure in patients with SSIs compared to controls [78]. In orthopaedic surgery, SSIs are also associated with increased amputation rates, and worse functional outcomes [57, 74, 76, 77, 79–81].
SSIs are defined as superficial incisional, deep incisional, or organ/space infections. A superficial incisional SSI occurs within 30 days, and involves only the skin and subcutaneous tissues. These type of SSI are usually treated with antibiotics alone, or possibly a minor debridement if there is dehiscence of the incision. A deep incisional or organ/space SSI is one that occurs within 30 days or alternatively within 1 year if implants are in place [82]. A deep SSI usually requires aggressive management and almost always some form of surgical debridement.
Orthopaedic spine surgery has an infection rate of about 2 % in elective cases, but has been reported up to 5 % at 1 year following surgery for traumatic spine injury [74, 77, 83]. Diabetes is strongly associated with SSI in these patients, with the risk being three times that of nondiabetic patients [74, 77, 83]. Furthermore, a preoperative HbA1c greater than 7.0 % is highly associated with SSI in spine surgery, with up to 35 % incidence in patients with uncontrolled DM [84]. Interestingly, increased serum glucose in patients without DM may also be an independent risk of SSI in spine surgery [74]. Correspondingly, in orthopaedic trauma, the stress-response increase in serum glucose also is associated with an increased risk of infection, even without history of diabetes [85–87]. Considering these studies, it appears that perioperative glycemic control is important to prevent SSI.
Periprosthetic infection (PPI) in total joint arthroplasty is also a serious epidemiologic issue. In 2003, there were about 200,000 total hip and 400,000 total knee arthroplasties performed in the USA. The projection to 2030 is a concerning 572,000 total hip and 3.5 million total knee arthroplasties annually [88]. A recent update seems to largely confirm those previous estimates [89]. In the general population, the arthroplasty infection rate is about 0.7 % [90]. Even this low rate of infection will generate a significant health burden of PPI, if the numbers of arthroplasty patients follows the predicted future trends.
Historically, rates of PPI in patients with diabetes have been as high as 7 % [76, 91]. An additional report has suggested a nearly fourfold increased risk of infection in those with diabetes [92]. However, a more recent report suggests a 1.2 times increase in the relative risk of PPI in diabetic patients [93]. Patients with uncontrolled DM preoperatively have a particularly high risk of PPI, urinary tract infection, transfusion, stroke, infection, and death [94]. In addition, even nondiabetic patients with a fasting preoperative glucose greater than 124 mg/dL have a significantly higher rate of PPI [95]. These studies strongly suggest preoperative DM optimization and glycemic control prior to orthopaedic procedures in order to prevent PPI and other complications.
Plantar Ulcerations
Plantar ulcers are a serious concern in patient with diabetes. Ulcers have predictive value for infection, sepsis, and amputation; and prophylactic care decreases patient morbidity and lowers expense of care [96]. The lifetime risk of plantar ulcer in diabetic patients has been estimated as high as 25 %, and the recurrence is 50 % at 3 years. In the past, up to 10 % of patients initially diagnosed with diabetes will have presented with an ulceration [97].
Peripheral neuropathy is the primary determinant of ulcer development, and 20–50 % of patients with diabetes have some degree of neuropathy [98–100]. With neuropathy, the loss of protective sensation in the foot allows an ulcer to start unchecked, as there is no painful feedback to the patient [101]. In addition, perhaps only half of patients with clinically significant diabetic neuropathy self-report neuropathic symptoms [98]. Therefore screening for sensation loss is the primary clinical method to determine those patients at risk for ulceration and to assign appropriate care measures accordingly [102, 103]. In addition to neuropathy, those patients with minor foot trauma and deformity are at very high risk of ulceration. The triad of neuropathy, trauma, and deformity is present in up to two thirds of patients with ulcers [103].
Screening has been suggested in the primary care setting, and is most commonly recommended with a Semmes-Weinstein 5.07 monofilament, which is a simple and cost-effective diagnostic strategy [96, 97, 100, 104, 105]. Additional screening methods are evaluation of vibratory sensation, temperature sensation, the presence of an Achilles tendon reflex, and transcutaneous oxygenation measurements [96, 102, 104–107]. Prevention is targeted at patient education, appropriate foot wear, optimization of blood glucose control, smoking cessation, identification of deformity and orthopaedic consultation, identification of large vessel disease and vascular consultation, and consistent follow-up care [96, 105].
Treatment of ulcers, once formed, is targeted to offload the ulcer with appropriate foot wear, orthotics, limited weightbearing, or total contact casting [108–111]. Additionally, optimization of blood glucose control, cessation of smoking, orthopaedic debridement or deformity correction, and vascular intervention complete the multidisciplinary approach to treatment of ulceration [112–116]. This treatment comes at a great cost, as the treatment of single foot ulcer over 2 years is estimated at around $28,000 [117, 118].
The time to successful healing of a plantar ulcer is usually on the scale of months, not days. The average healing time is variable, but many will heal by 2–3 months, with the more severe cases taking 4 months or longer [119, 120]. Most of the cross-sectional area of healing takes place at the beginning of treatment, but complete healing takes a much longer time [121]. The average time to healing is lower in those patients with smaller initial wounds, and those with more acute wounds [119, 120]. Transcutaneous oxygen tension may also predict ultimate healing potential [122]. Those ulcerations that ultimately are not able to be healed have very high incidences of osteomyelitis and eventual amputation. Of those diabetic patients who eventually end with amputation, up to 85 % had previous ulcerations [123–125]. In those ulcers with infection and ischemia, the outcomes are very poor. Patients with a deep wound, infection, and ischemia have an amputation rate of at least 50 %, and even a superficial wound can be 17–50 % [126].
Trigger Finger
Also known as stenosing tenosynovitis, trigger finger is an overuse disorder of the flexor tendon at the region of the A1 pulley. This pulley is the first in a set of pulleys in the finger that maintain the tendon–bone relationship while allowing the tendon to slide freely during movement [127]. The trigger finger is caused when there is a mismatch of tendon thickness to the internal diameter of the A1 pulley, and the tendon becomes stuck in the pulley after movement of the finger into flexion (Fig. 4.3) [127–129]. Patients commonly report using force to “pop” the finger back into extension.
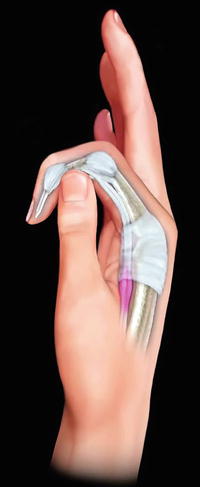

Fig. 4.3
Trigger finger
The incidence of trigger finger is 7–20 % of patients with diabetes comparing to only about 1–2 % in nondiabetic patients [130–132]. Trigger finger is also more common in women than men, except for a possible equal association in type I DM [133, 134]. When associated with diabetes, it commonly presents with multiple digit involvement, with a mean of 2.2 digits vs. 1.3 digits in nondiabetic patients [135, 136]. The duration of diabetes and insulin dependence is also associated with an increased incidence of trigger finger [130, 131, 134, 137]. However, the overall effectiveness of glycemic control does not seem to be correlated with the prevalence of trigger digit [131, 137].
Treatment of trigger finger is similar among patients with and without diabetes. Non-operative treatment includes stretching exercises to the flexor tendon, especially before and after activity, or injections of corticosteroid into the region around or inside the A1 pulley [138, 139]. Diabetic patients may have less successful non-operative treatment overall, especially if insulin dependent [136]. Finally, surgical treatment is generally reserved for patients who fail non-operative management, and is almost always a release of the A1 pulley. The pulley is not absolutely necessary for proper function of the fingers, and has a reported 97 % success rate [140].
Dupuytren’s Disease
Dupuytren’s disease (DD) is a hand disorder that is primarily symptomatic in older individuals, but can have relatively asymptomatic signs in younger patients as well. It was described by and named after Baron Dupuytren in the 1830s, but he was not the first to describe it [141]. The disease clearly has a genetic component, but with incomplete penetrance, and is most common in men and in patients of Northern European ancestry. It is also associated with a number of other factors including a history of trauma, epilepsy, alcoholism, smoking, adhesive capsulitis, and diabetes [142, 143].
Symptoms of DD begin with palmar nodules, and later with pretendinous cords. Later, the cords begin to contract and cause flexion of the digits, limiting extension and causing flexion contractures (Fig. 4.4). These contractures are frequently the presenting symptoms, as they have a direct limitation of patient activity and overall hand function. The cords contain myofibroblasts and organize along predictable, but abnormal routes in the hand and fingers [144, 145]. Most often, the ring and small fingers are involved in nondiabetic patients, but any digit can be affected. Curiously, diabetic patients with DD are more likely to have long and ring finger involvement instead of ring and small finger involvement [133]. Also, the symptoms of the disease tend to be milder, and progression is slower in those diabetic patients than the general population [141]. Although men are more prone to develop the disease, diabetic women have a high chance of also being affected [133, 146].
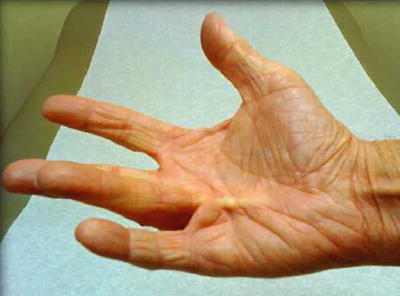

Fig. 4.4
Dupuytren’s disease
The prevalence of DD in the general population is estimated to be 1–8 %, but depends on the diagnostic criteria used. Those patients who present with cords may be closer to 1 %, but those with palmar nodules are probably closer to 7–11 % of the population [130, 133]. The risk of developing Dupuytren’s disease in diabetic patients is significantly higher than that of general population at between 19 and 42 % [130, 133, 141]. Dupuytren’s disease has been reported to have incidence rates in type I diabetes patients comparable to type II diabetes patients, and correlates to the duration of diabetes [133, 134, 147, 148]. Impaired glycemic control as measured by HbA1c, mean glucose, and presence of diabetic comorbidities seem to correlate with the prevalence of DD [130, 141].
Noninvasive treatment is largely unsuccessful in improving the features of the disease. One minimally invasive treatment option is injection of collagenase followed by manipulation of the digits, and this method has been shown to be an effective treatment [149]. Percutaneous release of the cords is another treatment option that does not have the risk of wound complications postoperatively compared to open fasciectomy, but may not have as significant a correction overall [150, 151]. Open surgical treatment has historically been a complete or partial fasciectomy, which is aggressive removal of the cords of abnormal tissue. Complications of partial fasciectomy are incomplete correction, recurrence, infection, and wound problems. Even with surgical treatment, however, recurrence is up to 80 %, but only about 12 % have additional treatment from loss of function or recurrence [146]. Surgical treatment in diabetic patients does not seem to have an increased risk of complication [152].
Carpal Tunnel Syndrome
Carpal tunnel syndrome (CTS) is the compression of the median nerve within the carpal tunnel in the wrist, and it classically manifests as pain and paresthesias of the thumb, index, long, and the radial half of the ring finger. Symptoms are usually worse at nighttime, and patients frequently report being woken up from numbness in the hand. CTS is typically an overuse injury related to occupational and daily activities, and is more common in women [131, 153–155]. However, it can also present in cases with extrinsic compression of the carpal tunnel from masses, or acutely from trauma.
The prevalence of CTS in patients with diabetes has been estimated at 11–30 % [130, 133, 153, 156], and is dependent on the duration of diabetes. In contrast, the incidence is estimated at 2–3.8 % of the general population [155, 157]. Bilateral involvement of CTS is common in diabetic patients, but both sides are not necessarily symptomatic simultaneously. Type I DM patients have a high prevalence of CTS with increasing duration of disease, up to 85 % after 54 years of DM. However the prevalence does not seem to be associated with glycemic control [130, 158].
Overall, the basic science mechanism in which DM is associated with CTS is not well understood, but microangiopathy has been suggested as a likely contributing factor [159, 160]. However, nephropathy, retinopathy, and polyneuropathy have not been consistently identified as risk factors for CTS, possibly from the confounding effect of duration of diabetes [130, 157, 158].
Initial treatment of CTS is generally non-operative and initiated with splinting, especially at night. Injections into the carpal tunnel are sometimes advised in milder disease, and can sometimes be effective long-term [161]. Surgical treatment consists of a release of the transverse carpal ligament, which decompresses the median nerve and is a highly effective treatment of patients’ symptoms [160, 162]. However, there may be a slightly less efficacious outcome in patients with DM [159, 160].
Adhesive Capsulitis
Adhesive capsulitis, also known as frozen shoulder, is a disorder of the shoulder characterized by pain and stiffness [163], and is commonly arbitrarily divided into phases [164–166]. The first phase is painful, without significant loss of shoulder range of motion. The second phase remains painful, but the shoulder becomes significantly stiffened. In the third phase, the shoulder remains stiff, but is less painful. Finally, the fourth phase is spontaneous resolution, which is sometimes mistakenly believed to happen in all patients [164, 165, 167].
Adhesive capsulitis is a term coined by Nevassier for a disorder of the shoulder in which the glenohumeral joint capsule thickens and has an adhesive appearance when opened to be released [163]. Although used interchangeably, frozen shoulder describes the clinical presentation of a stiff shoulder, either from a primary condition of capsular thickening or a secondary cause from another shoulder diagnosis such as rotator cuff pathology. Adhesive capsulitis is used as a description of the pathological condition of capsular thickening and hypertrophy resulting in shoulder stiffness [163, 164].
Adhesive capsulitis is associated with a number of other medical conditions including diabetes, both type 1 and 2 [166–171]. Other associated conditions reported are trauma, cervical spine disease, Dupuytren’s disease, coronary heart disease, tuberculosis, carcinoma, hyperthyroidism, and epilepsy. However, the relationship to these medical comorbidities is unclear, including diabetes [171]. The incidence of adhesive capsulitis is up to 11–20 % in diabetic patients, in comparison to 2–5 % of control patients [171, 172]. Serum glycosylated hemoglobin levels have been studied, but the levels do not seem to correlate with a risk for adhesive capsulitis [166, 168]. Supporting this finding, histologically measured glycosylation of tissues does not seem to directly correlate with the risk of other secondary complications of diabetes including joint stiffness [173]. In type I diabetic patients, the prevalence of adhesive capsulitis is increased after 20 years duration of the disease process [168, 174]. Type II patients do not have a measurable association with duration of disease [168]. However, patients in the past were not commonly diagnosed with DM until years after the process actually commenced. DM patients with a history of myocardial infarction [168, 171] and autonomic neuropathy have been independently associated with an increased risk of adhesive capsulitis [168].
Diagnosis of adhesive capsulitis is based predominately on a loss of passive range of motion in a shoulder, and the exclusion of other diagnoses that may also cause a secondary loss of range of motion from pain [167, 171, 175]. A diagnostic injection into the subacromial space may aid in diagnosis to eliminate pain from other coexisting diagnoses such as rotator cuff pathology, as even in those cases the shoulder range of motion is limited. Range of motion in adhesive capsulitis is limited in multiple directions, but especially in external rotation and abducted external rotation [167]. This ROM can be compared against the contralateral side to determine a patient’s normal ROM, as it can differ among patients [176].
The natural history of adhesive capsulitis has commonly been cited as a self-limiting process. This citation would suggest that the condition resolves on its own, but careful review of past literature would suggest otherwise. Up to 60 % of patients followed for an average 7 years have some persistent symptoms and disability [177]. In addition, about 50 % of patients have incomplete resolution of symptoms at 1 year [164, 165, 167]. These persistent symptoms support early treatment in most patients.
Treatment of adhesive capsulitis is overall similar in diabetic and nondiabetic patients; however, the results are worse in diabetic patients. Diabetic patients have less range of motion, more pain, and more disability after treatment [169, 170]. Treatment options include physical therapy, intra-articular corticosteroid injection, manipulation under anesthesia, and surgical release [178].
One study of aggressive vs. gentle ROM would suggest that gentle ROM may be better than aggressive manipulation [179]. However, it has been unclear which intervention or combination of interventions are the most successful at treatment [180–182]. Corticosteroid injection may improve with pain, but it is unclear if the long-term outcomes are changed [165, 181, 182]. Oral corticosteroid does not appear to affect outcomes positively [165, 183, 184].
Manipulation under anesthesia is indicated in some cases that have failed a course of physical therapy [175]. This manipulation can be under regional or general anesthesia, and may be followed or preceded with an intra-articular injection of steroid solution. Follow-up with physical therapy immediately afterwards is considered standard. A complication to be avoided is fracture of the humerus, which if occurs, may require additional surgical fixation to continue physical therapy, as immobilization would likely result in treatment failure.
Finally, arthroscopic capsular release is indicated in those patients with 3–6 months of failure of other non-operative options, especially those with significantly stiff shoulders. The result of capsular release is marginally improved over manipulation, but is considered in refractory cases [185]. Risks of the procedure include fracture and axillary nerve injury, where the nerve is located in the axillary recess, immediately underneath the capsular tissue.
Conclusion
Diabetes mellitus is a significant medical issue that increases the risk of certain orthopaedic conditions and complications, and leads to less successful outcomes than in nondiabetic patients. Glycemic control is certainly recommended before any elective procedure, and has become more recognized to be a primary goal after traumatic injuries as well.
Hip fractures are a varied group of fractures around the hip, of which DM significantly increases the risks fracture, the risks of complications, and the risk of mortality after treatment. Ankle fractures are a major source of complications in patients with diabetes after treatment, both non-operatively and operatively. Overall, operative treatment of unstable ankle fractures is superior to that of non-operative treatment. Some patients will ultimately end with amputation despite best efforts.
Infection risk is increased postoperatively after orthopaedic procedures, and there is an increasing emphasis on glycemic control perioperatively to reduce the risk of SSI. Plantar ulcerations are largely a complication of diabetic neuropathy, deformity, and minor trauma. Prevention is more cost-effective than treatment, as the presence of an ulcer is a predictor of ultimately poor outcomes.
Trigger finger has a high incidence in patients with DM, and more fingers are generally involved at presentation in patients with DM. Treatment options are no different than the general population, but successful treatment may be slightly less efficacious in patients with DM than in nondiabetic patients. Dupuytren’s disease has a high prevalence in patients with DM, and there is a predilection for long finger and ring finger involvement rather than the small and ring finger involvement seen in the general population. DD is associated with the duration of diabetes, but the presentation in DM patients is less severe on the whole. The treatment of DD is similar in patients with or without DM. Carpal tunnel syndrome is a common orthopaedic hand issue in patients with diabetes, at least four times the general population incidence, and is associated with the duration of diabetes. The incidence of CTS may be even higher in type I DM than in type II DM. Treatment of CTS in DM and nondiabetic patients is not significantly different than the general population. Surgical treatment is usually successful, but possibly less improvement in patients with DM. Adhesive capsulitis has a high incidence in DM patients as well. The overall presentation is similar, but the treatments may be less successful overall. After treatment, patients with DM have more pain and stiffness on average vs. patients without DM.
Diabetic-associated orthopaedic conditions and complications are commonly seen in the general orthopaedic practice, and are challenging to treat. Although some of the conditions associated with DM do not depend on glycemic control, most orthopaedic complications of treatment do have increased risks in those patients with either acutely or chronically poor blood glucose control. In the future, the medical maintenance of DM and attention to perioperative glycemic control may be the most effective interventions to limit these set of orthopaedic complications in patients with DM.
References
1.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–46.PubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree