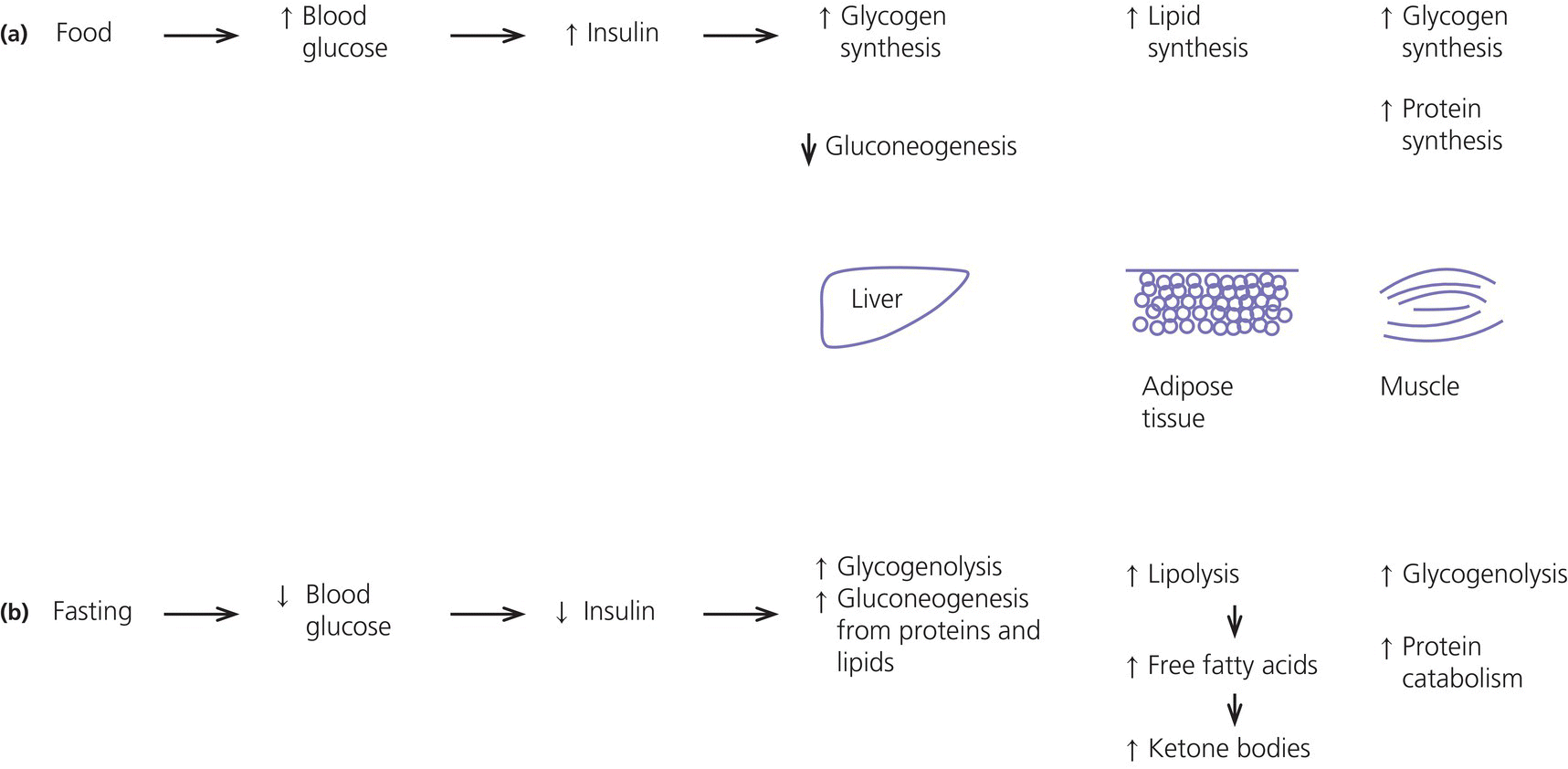
1 Diabetes mellitus is a heterogeneous disorder characterized by abnormal metabolism of carbohydrate, fat and protein with persistent fasting or postprandial hyperglycaemia resulting from defects in insulin secretion or insulin action (Skyler et al. 2017). It is diagnosed in one of four ways (see Table 1.1) (American Diabetes 2018). A fasting plasma glucose (PG) of 5.6–6.9 mmol/L (100–125 mg/dL) is considered prediabetes, whereas <5.6 mmol/L (<100 mg/dL) is normal. The oral glucose tolerance test (OGTT) is not recommended for routine clinical use. When classic symptoms are present, the diagnosis is usually straightforward and an OGTT is seldom needed; however, an OGTT may be indicated when mild hyperglycaemia is discovered without symptoms (e.g. in the sibling of a child with diabetes or in children with disorders such as cystic fibrosis (CF) that predispose to diabetes and may be asymptomatic in the early stages). Table 1.1 Criteria for the diagnosis of diabetes mellitus. Definitions are based on venous plasma glucose levels. Glucose meters are useful for screening in clinics and physicians’ offices, but the diagnosis of diabetes mellitus must be confirmed by measurement of venous plasma glucose on an analytic instrument in a clinical chemistry laboratory. In the absence of unequivocal hyperglycaemia, criteria 1 – 3 should be confirmed by repeat testing on a different day. Fasting is defined as no caloric intake for at least eight hours. b OGTT should be performed using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water. Haemoglobin A1c test should be performed in a laboratory using a method certified by the National Glycohemoglobin Standardization Program (www.ngsp.org). The incidental discovery of hyperglycaemia without classic symptoms does not necessarily indicate new onset diabetes, especially in young children with an acute illness, who may experience ‘stress hyperglycaemia’. The risk of eventually developing diabetes may be increased in some children with incidental or stress hyperglycaemia, especially those with immunologic, metabolic, or genetic markers for type 1 diabetes, and consultation with a paediatric endocrinologist is indicated. Diabetes mellitus is classified on the basis of its pathogenesis (Table 1.2); it may be the result of severe insulin deficiency or insulin resistance or, more commonly, a combination of milder defects in insulin secretion and action (American Diabetes Association 2018). This chapter primarily focuses on type 1 diabetes, which is the commonest form of diabetes in children. Other causes of diabetes are discussed in Sections 1.21 and 1.24. Table 1.2 Protocol for and interpretation of the oral glucose tolerance test. An OGTT should be performed after at least three days of adequate carbohydrate consumption (≥150 g per 1.73 m2) and is performed using 1.75 g/kg anhydrous glucose dissolved in water for individuals ≤43 kg and 75 g for weight > 43 kg. The incidence of type 1 diabetes in children varies considerably across the world with the Scandinavian countries having the highest incidence; in Finland, 60 new cases per 100 000 children under 15 years of age. The United Kingdom, Canada, the US, and Australia also have high incidences with more than 20 cases per 100 000 children, whereas Asia and Sub‐Saharan Africa have much lower rates (China and India 0.1 cases per 100 000 people each year) (Patterson et al. 2014). The reasons for these large variations are unclear but may include genetic factors given the evidence of variations in the incidence of diabetes in different ethnic groups (e.g. in the US, the incidence is higher in non‐Hispanic white than African‐American or Hispanic youth). However, this difference cannot solely be attributed to genetic factors. In Europe, the risk of type 1 diabetes differs substantially in people who are genetically close but separated by socio‐economic borders. Furthermore, over the past 30 years, the worldwide incidence has steadily increased across all age groups in parallel with an increased standard of living. A European population‐based registry showed a 3.9% annual increase in the incidence of type 1 diabetes in children <15 years between 1989 and 2003 (5.4% in the 0–4 year age group) and the US population‐based SEARCH for Diabetes in Youth study has shown that the prevalence in people <20 years increased by 21% between 2001 and 2009. With some exceptions, type 1 diabetes incidence is related to geographic distance north of the equator, and the onset of disease appears to be higher in autumn and winter than in spring and summer. Table 1.3 presents the American Diabetes Association Classification of Diabetes. Table 1.3 The American Diabetes Association classification of diabetes. Type 1 diabetes is a chronic autoimmune disease caused by an incompletely understood complex interaction between risk‐conferring genes and environmental factors resulting over time (years) in immune‐mediated, selective destruction and loss of function of pancreatic β‐cell mass. This leads to insulin deficiency, symptoms from hyperglycaemia, and lifelong insulin dependence (Insel et al. 2015). Symptoms occur when approximately two‐thirds of the pancreatic islets are devoid of ß‐cells. Although 90% of patients with type 1 diabetes do not have a family history of the disease, development of type 1 diabetes is strongly influenced by genetic factors. Children born into families with type 1 diabetes have different lifetime risks depending on whether the mother (6%), father (12%), or a sibling (5–10% by age 20 years) has the disease (Pociot and Lernmark 2016). If a twin develops type 1 diabetes, the lifetime risk for the non‐affected dizygotic twin is 6–10%, whereas that for a monozygotic twin is approximately 60%. Type 1 diabetes is a polygenic disorder; more than 50 susceptibility loci that contribute to the likelihood of developing type 1 diabetes have been identified. The major histocompatibility complex (MHC) region encoding the human leukocyte antigen (HLA) on chromosome 6p21 (the IDDM1 locus) contributes about 50% of the genetic risk. The insulin gene locus (INS) is the second most important susceptibility locus, contributing about 10% of genetic susceptibility. Each of the loci identified through genome‐wide association studies has a slight individual effect on the total genetic risk for progression to type 1 diabetes, and gene variants collectively explain ~80% of type 1 diabetes heritability (Pociot and Lernmark 2016). Most of the loci associated with risk of type 1 diabetes are thought to involve immune responses (Concannon et al. 2009), supporting the notion that genetic influences involve mechanisms that collectively contribute to aberrant immune responsiveness. Genetic susceptibility might also influence responses to environmental stimuli, modify viral responses or physiological pathways. For most of the genetic loci, however, the molecular mechanism of action remains unknown. Newborn screening has been used to identify children at increased genetic risk who have been followed for the appearance of autoantibodies against ß‐cell autoantigens: insulin, glutamic acid decarboxylase (GAD), insulinoma‐associated antigen‐2 (IA2), and zinc transporter 8 (ZnT8A) that are known to be strongly associated with an increased risk for type 1 diabetes. These autoantibodies can appear as early as age six months, with a peak incidence in the second year of life in genetically susceptible individuals, i.e. they are present months to years before the onset of symptoms. Children who develop two or more islet autoantibodies have a markedly increased likelihood of eventually developing type 1 diabetes, and 100% of those who develop a third and, often, a fourth autoantibody develop clinical type 1 diabetes when followed for 20 years (Ziegler et al. 2013). At the time of diagnosis, more than 90% of individuals with type 1 diabetes have at least one autoantibody, and the presence of autoantibodies against ß‐cell autoantigens is a key feature distinguishing type 1 from type 2 diabetes. Type 1 diabetes is also associated with other autoimmune disorders, most commonly autoimmune thyroiditis. At the time of diagnosis, about 25% of children have thyroid autoantibodies, which predict thyroid dysfunction (most commonly hypothyroidism); Graves’ disease occurs in ~0.5% of patients with type 1 diabetes. Addison’s disease, likewise, occurs in approximately 0.5% of patients with type 1 diabetes. Coeliac disease is another immune‐mediated disorder that occurs with increased frequency in patients with type 1 diabetes, and biopsy‐confirmed coeliac disease occurs in 3.5% of individuals with type 1 diabetes compared with 0.3–1% in the general population. The increase in incidence described above can only be explained by changes in environment or lifestyle and it is notable that migrants tend to acquire the same risk of type 1 diabetes as the native population in their new area of residence (Rewers and Ludvigsson 2016). Studies of prospective birth cohorts are attempting to identify potential triggers of islet autoimmunity and the natural history of progression to diabetes. Putative triggers include infections, diet, and toxins that affect children in utero, perinatally, or during early childhood. See Rewers and Ludvigsson (2016) for a review of this topic. There has been speculation that vaccines might trigger autoimmunity; however, no association has been detected with islet autoimmunity or type 1 diabetes, and a recent meta‐analysis concluded that childhood vaccines do not increase the risk of type 1 diabetes (Morgan et al. 2016). Some forms of type 1 diabetes have no known aetiology. Most patients with idiopathic type 1 diabetes are of African or Asian ancestry. They have permanent insulinopenia and are prone to episodic ketoacidosis, but have no evidence of beta‐cell autoimmunity (negative islet autoantibodies). This form of diabetes is strongly inherited but not HLA‐associated. Between episodes, patients exhibit varying degrees of insulin deficiency and may intermittently need insulin replacement. Insulin is an anabolic hormone that acts on liver, fat, and skeletal muscle to increase glucose uptake, oxidation, and storage, and to decrease glucose production. Insulin also inhibits lipolysis and thereby limits the availability of fatty acids for oxidation and inhibits ketogenesis. Insulin is secreted in two major patterns – basal and in response to food (prandial). Basal secretion produces relatively constant, low plasma insulin levels that restrain lipolysis and hepatic glucose production (from glycogenolysis and gluconeogenesis). The blood glucose concentration is the dominant stimulus for insulin secretion. After a meal, in parallel with the rise in plasma glucose, circulating insulin concentrations rise rapidly, facilitating the entry of glucose into cells via glucose‐specific transporters, particularly in skeletal muscle and adipose tissue. Insulin stimulates glycogen synthesis in the liver and skeletal muscle, inhibits hepatic gluconeogenesis, and stimulates fat storage and protein synthesis. Conversely, during fasting, plasma glucose concentrations and insulin secretion decrease, leading to reduced glucose uptake in muscle and adipose tissue, increased lipolysis, and stimulation of hepatic glucose production (from glycogenolysis and gluconeogenesis (Figure 1.1). Figure 1.1 Glucose homeostasis: a comparison of (a) the fed state and (b) the fasting state. In type 1 diabetes, insulin deficiency results in hyperglycaemia and when the plasma glucose concentration exceeds the renal threshold for glucose reabsorption (~180 mg/dL or 10 mmol/L) osmotic diuresis occurs, causing polyuria and polydipsia. Insulin deficiency also causes increased lipolysis with the production of excess free fatty acids and ketoacids (beta‐hydroxybutyrate (BOHB) and acetoacetate) leading to hyperketonaemia and ketonuria. When fluid losses exceed intake, particularly when nausea and vomiting occur (typical symptoms of ketosis), dehydration develops. The accumulation of ketoacids in the blood causes metabolic acidosis, which results in compensatory rapid, deep breathing (Kussmaul respiration). Acetone, formed from acetoacetate, accounts for the characteristic smell of the breath (described as the odour of nail polish remover or rotten fruit). Accompanying the lack of insulin is an increase in the levels of stress or counter‐regulatory hormones (glucagon, catecholamines, cortisol, and growth hormone) whose metabolic actions are opposite to those of insulin. Thus, a lack of insulin together with increased concentrations of counter‐regulatory hormones leads to progressive hyperglycaemia, hyperfattyacidemia, and ketosis and eventually ketoacidosis. Progressive dehydration, acidosis, and hyperosmolality cause decreased consciousness and lead to coma and death if untreated. At diagnosis, typical symptoms have usually been present for only a few days to about two weeks or longer especially in type 2 diabetes: Although most school‐aged children report polyuria and polydipsia, these symptoms may be less obvious in the very young child (e.g. an infant in diapers) in whom the other less characteristic symptoms, especially perineal candidiasis, may predominate. Clinical manifestations of diabetic ketoacidosis (DKA) include: Note that symptoms of systemic infection are infrequent; however, one must carefully look for an infection, especially if there is fever. Patients with DKA typically are dehydrated. Clinical estimation of the degree of dehydration is imprecise and generally shows only fair to moderate agreement among examiners. The most useful signs for predicting 5% dehydration in young children are: Other useful signs to assess degree of dehydration include: dry mucous membranes, sunken eyes, absent tears, weak pulses, and cool extremities. More signs of dehydration tend to be associated with more severe dehydration; ≥10% dehydration is suggested by the presence of weak or impalpable peripheral pulses, hypotension, cool extremities, and oliguria. Children diagnosed with diabetes should be immediately referred to a hospital for evaluation and management. The diagnosis of type 1 diabetes usually is obvious because the patient has classical symptoms (polyuria, polydipsia, and weight loss), random blood glucose is >11 mmol/L (200 mg/dL) and there is glucosuria with or without ketonuria. Diabetes should also be considered in the differential diagnosis of any child presenting with impaired consciousness and/or acidosis. Tachypnea and hyperventilation in DKA may lead to the erroneous diagnosis of pneumonia or bronchiolitis. The lack of a cough or wheeze and the absence of abnormal findings on auscultation of the chest and a normal chest radiograph should raise the possibility of a metabolic acidosis such as DKA as the cause of tachypnea. Abdominal pain and tenderness in DKA may suggest a surgical emergency such as appendicitis or acute pancreatitis. Fluid, electrolytes, and insulin therapy will ameliorate the abdominal symptoms within hours. Diabetes should always be considered in children with secondary nocturnal enuresis and those with recurrent or persistent perineal candidiasis. Acute illnesses such as severe sepsis or a prolonged convulsion may, occasionally, cause hyperglycaemia, glycosuria, and ketonuria. However, these biochemical abnormalities are almost always transient and are rarely associated with a history of previous polydipsia and polyuria. If in doubt, a fasting blood glucose measurement or OGTT (see Table 1.1) should be performed. A doctor who either suspects or has made a definitive diagnosis of diabetes should immediately refer the child to a specialist for comprehensive assessment and initiation of treatment. At diagnosis, perform the following investigations: The criteria for diagnosis of DKA are: plasma glucose ≥200 mg dL (11.1 mmol/L), venous pH < 7.30 or serum bicarbonate <15 mmol/L and ‘moderate’ or ‘large’ ketonuria, or serum BOHB ≥3 mmol/L (Wolfsdorf et al. 2018). The goals of initial management of the child with newly diagnosed diabetes mellitus are to restore the fluid and electrolyte balance, to stabilize the metabolic state with insulin, and to provide basic diabetes education and self‐management training for the child (when age and developmentally appropriate) and caregivers (parents, grandparents, guardians, older siblings, daycare providers, and babysitters). The diagnosis of diabetes in a child is a crisis for the family, who require considerable emotional support and time for adjustment and healing. Shocked, grieving, and overwhelmed parents require time to acquire basic (‘survival’) skills while they are coping with the emotional upheaval that typically follows the diagnosis of diabetes in a child. Even if they are not acutely ill, and depending on local resources and practices, children with newly diagnosed type 1 diabetes may be admitted to hospital to initiate insulin treatment and for diabetes education and self‐management training. Outpatient or home‐based management is preferred by some centres that have the requisite resources, in particular, the availability of travelling diabetes nurses who will need to visit at least daily in the first few days and maintain regular telephone contact, often outside normal working hours (Lowes and Gregory 2004). The size of the geographical area that needs to be covered is an important consideration. Outpatient or home‐based management may have several advantages: the stress of a hospital stay is avoided, the outpatient setting or patient’s home is a more natural learning environment for the child and family, and ambulatory treatment reduces the cost of care. Where adequate outpatient and/or home initial management of type 1 diabetes at diagnosis can be provided, studies have shown there is no disadvantage in terms of metabolic control nor increase in acute complications, hospitalizations, psychosocial or behavioural problems or total costs. The decision concerning whether a child with newly diagnosed diabetes should be admitted to hospital depends on several factors: most important are the severity of the child’s metabolic derangements, the family’s psychosocial circumstances, and the resources available at the treatment centre. Many paediatric diabetes centres offer ambulatory care and provide diabetes education and training in a day care unit for several days following diagnosis. Hospital admission is necessary if intravenous (IV) therapy is required to correct dehydration, electrolyte imbalance, and ketoacidosis or if there are psychosocial challenges. Children who are ≤5% dehydrated, not nauseous or vomiting, who are not particularly unwell, and have a pH ≥7.30 usually respond well to subcutaneous insulin and oral rehydration. Optimal care of children with type 1 diabetes is complex and time‐consuming. Children with diabetes should be managed by a multidisciplinary diabetes team, which provides diabetes education and care in collaboration with the child’s primary care physician. The team should consist of a paediatric endocrinologist or paediatrician with training in diabetes management, a paediatric diabetes nurse educator (DNE) or diabetes nurse specialist (advanced practice nurse), a dietitian trained in paediatric diabetes nutrition, and a mental health professional (a clinical psychologist or medical social worker). The diabetes team should always be available by telephone to provide guidance and support to parents and patients and to respond to metabolic crises that require immediate intervention. Education is the foundation of diabetes care and is vital to ensure successful outcomes. Diabetes education provides the knowledge and skills needed to perform diabetes self‐care and make the lifestyle changes required to successfully manage the disease. The diabetes education curriculum should be adapted to the individual child and family. Parents and children with newly diagnosed diabetes are usually anxious and overwhelmed and frequently cannot assimilate a large amount of abstract information. Therefore, the education programme should be staged. Initial educational goals should be limited to basic skills so that the child can be safely cared for at home and return to his or her daily routine as soon as possible. Initial diabetes education and self‐management training should include: understanding what causes diabetes, how it is treated, how to measure and administer insulin, basic concepts of meal planning, self‐monitoring of blood glucose (SMBG) and ketones, recognition, and treatment of hypoglycaemia, and how and when to contact a member of the diabetes team for advice. If several members of the diabetes team are involved in educating the newly diagnosed child and his or her family, good communication between team members to ensure consistency in the messaging and the specific information given is important. The following topics should be included in the curriculum and discussed with the child and family over a period of several weeks or months following diagnosis: The child’s primary care physician should be informed of the child’s diagnosis, management plan, and discharge from hospital, and the diabetes nurse should communicate with the school nurse or daycare facility to ensure that details of the care plan are in place and understood. The equipment that a child will need on discharge is shown in Table 1.4. Table 1.4 Supplies required at time of discharge. When the child is medically stable and parents (and other care providers) have mastered basic diabetes management skills, the child is discharged from the hospital or ambulatory treatment centre. In the first few weeks after diagnosis, frequent telephone contact provides emotional support and helps parents to interpret the results of blood glucose monitoring and, when necessary, insulin doses are adjusted to achieve blood glucose levels in a defined target range. Within a few weeks of diagnosis, many children enter a partial remission (the ‘honeymoon’ phase), evidenced by normal or near‐normal blood glucose levels on a low dose (<0.25 U/kg/day) of insulin. By this time, most patients and parents are less anxious, have mastered basic diabetes management skills through repetition and experience, and are now more prepared to begin to learn the intricate details of intensive diabetes management. At this stage, the diabetes team should begin to provide patients and parents with the knowledge they will need to maintain optimal glycaemic control while coping with the effects of exercise, varying food intake, intercurrent illnesses, and the other challenges that normally occur in a child’s daily life. In addition to teaching facts and practical skills, education should promote desirable health beliefs and attitudes in the young person with a chronic incurable disease. For some children, this may be best accomplished in a non‐traditional educational setting, such as a summer camp for children with diabetes. The educational curriculum must be concordant with the child’s level of cognitive development and has to be adapted to the learning style and literacy and numeracy skills of the individual child and family. Parents, grandparents, older siblings, the school nurse, and other important people in the child’s life are encouraged to participate in the diabetes education programme so they can actively share in the diabetes care and ensure that the child with diabetes is not excluded from normal childhood activities (sports, field trips, sleepovers, etc.). In the first month after diagnosis, the patient and care providers are seen frequently by the diabetes team to review and consolidate the diabetes education and practical skills learned in the first few days and to extend the scope of diabetes self‐management training. Thereafter, follow‐up visits with members of the diabetes team should occur at least every three months. Regular clinic visits are necessary to ensure that the child’s diabetes is being appropriately managed and the goals of therapy are being met. A focused history should obtain information about self‐care behaviours, the child’s daily routines, the frequency, severity, and circumstances surrounding hypoglycaemic events, details about insulin doses, and blood glucose monitoring data should be reviewed to identify patterns and trends. At each visit, height and weight are measured and plotted on a growth chart. The weight curve is especially helpful in assessing the adequacy of therapy. Significant weight loss usually indicates that the prescribed insulin dose is insufficient or the patient is not receiving all the prescribed doses of insulin. A complete physical examination should be performed at least once or twice each year focusing on blood pressure, stage of puberty, evidence of thyroid disease, examination of the injection sites for evidence of lipohypertrophy (from over‐use of the site) or lipoatrophy, and mobility of the joints of the hands. Each clinic visit provides an opportunity to reinforce the individual patient’s blood glucose targets and HbA1c goal, and to increase the patient’s and the family’s understanding of diabetes management, the interplay of insulin, food, and exercise, and their impact on blood glucose levels. As the child’s cognitive development progresses, the child should become more involved in diabetes management and increasingly assume supervised age‐appropriate responsibility for daily self‐care. Parents are encouraged to contact the diabetes team for advice if the pattern of blood glucose levels changes between routine visits, suggesting the need to adjust insulin doses or change the regimen. Eventually, when parents and patients have sufficient knowledge and experience to interpret blood glucose patterns and trends, they are encouraged to independently adjust insulin doses. The diagnosis of diabetes in a child or adolescent hurls parents into a frightening and foreign world. They grieve the loss of their healthy child and have to cope with normal distress reactions, including shock, disbelief and denial, fear, anxiety, anger, and blame or guilt. During this emotionally intense time, parents are expected to rapidly acquire an understanding of the disease and manage the illness at home. Parents should receive the necessary support to begin coping with their emotional distress and not be overwhelmed by unrealistic expectations from a well‐meaning diabetes treatment team. Diabetes also presents family members with the task of being sensitive to the balance between the child’s need for a sense of autonomy and mastery of self‐care activities and the need for ongoing family support and involvement. The struggle to balance independence and dependence in relationships between the child and family members presents a long‐term challenge and raises different issues for families at different stages of child and adolescent development. Focusing on normal developmental tasks at each stage of the child’s growth and development provides the most effective structure to address this concern (Anderson et al. 2009). A medical social worker or clinical psychologist should perform an initial psychosocial assessment of all newly diagnosed patients to identify families at high risk who need additional services. Thereafter, patients are referred to a mental health specialist when emotional, social, environmental, or financial concerns are suspected or identified that interfere with the ability to maintain acceptable diabetes control. Some of the more common problems in families who have a child with diabetes include parental guilt, resulting in poor adherence to the treatment regimen, difficulty coping with the child’s frustration and rebellion against treatment, fear of hypoglycaemia, anxiety, depression, missed appointments, financial hardship, or loss of health insurance affecting the ability to attend scheduled clinic appointments and/or purchase supplies. Patients with poor glycaemic control and a history of frequent emergency department visits should be screened for depressed mood (Lawrence et al. 2006). Recurrent ketoacidosis is the most extreme indicator of psychosocial stress, and management of such patients must include a comprehensive psychosocial assessment. Because childhood is characterized by cognitive and emotional immaturity, successful treatment of paediatric diabetes requires the continuous, active involvement of responsible adults. Moreover, diabetes treatment occurs within a family dynamic, and treatment‐related conflicts are common, arising in part from a natural discord in goals between caretakers and the child. Each phase of childhood has unique characteristics that complicate treatment; for example, the normally erratic eating behaviour of toddlers and the unscheduled intense physical play of school‐aged children that can hinge on unpredictable factors, such as the weather. Adolescence is characterized by multiple physiologic and psychosocial factors that make glycaemic control even more challenging. Diabetes treatment should be individually tailored to each child, based on age, family resources, cognitive ability, the schedule and activities of the child and family, and their goals and desires. Rates of psychological ill health in youth with diabetes are high, and longitudinal data indicate that mental health issues in childhood are likely to persist into early adulthood and are prognostic of maladaptive lifestyle practices, long‐term problems with diabetes control and earlier‐than‐expected onset of complications. For these reasons, mental health screening should be routinely performed in diabetes clinics. Screening for behavioural disturbance should begin in children at the time of diagnosis, with further assessment of parental mental health and family functioning for those children identified to be ‘at risk’. Interventions can then be targeted based on the specific needs of individual children and families. Additional psychological support is often provided by diabetes nurses and other parents at local and national support groups. This clinical trial, completed in 1993, proved that near‐normal glycaemia delays the onset and slows the progression of microvascular complications, and it set the current standards for treatment of type 1 diabetes. A total of 1441 subjects with diabetes aged 13–39 years were randomized either: (i) to continue with their conventional treatment; or (ii) to receive intensive therapy with increased support from the ‘diabetes team’ and insulin administered either by three or more injections daily or by a pump (The Diabetes Control and Complications Trial Research Group 1993). After a mean duration of 6.5 years, as compared with conventional therapy, intensive treatment resulted in a reduction in: For every 10% reduction in HbA1c (e.g. 8% vs. 7.2%), there was a 44% reduction in the risk of microvascular complications. Intensive treatment using the insulin preparations available at the time (i.e. before the development of insulin analogues) was associated with a two‐to‐threefold increase in severe hypoglycaemia and a mean weight gain of 4.6 kg when compared with conventional treatment. This study clearly demonstrated that near‐normal glycaemia (as measured by HbA1c) significantly reduced the risk of microvascular complications. In the 25 years since the results of this landmark study were announced, the challenge for clinicians has been to implement the principles of intensive diabetes therapy in children and adolescents in routine clinical practice. The International Society for Pediatric and Adolescent Diabetes (ISPAD) recommends a target HbA1c of <7.5% (58 mmol/mol) for all age groups (DiMeglio et al. 2018). However, biochemical goals should be individualized, taking into account both medical and psychosocial considerations; i.e. each child should have individually determined targets with the goal of achieving an HbA1c value as close to normal as possible while avoiding frequent episodes of mild to moderate hypoglycaemia and severe hypoglycaemia. Less stringent treatment goals may be appropriate or more realistic for preschool‐aged children, children with developmental handicaps, psychosocial challenges, lack of appropriate family support, children who have experienced severe hypoglycaemia, or those with hypoglycaemia unawareness. At the time of diagnosis, many children with type 1 diabetes are severely insulin‐deficient and require insulin replacement to survive. The aim of insulin replacement therapy is to simulate normal plasma insulin patterns as closely as possible. Truly physiologic insulin replacement continues to be an elusive goal owing to: (i) delivery of insulin into the systemic circulation instead of the portal venous system, and (ii) the inability to mimic the first and second phases of normal insulin release in response to eating. Insulin pump therapy or multiple daily insulin (MDI) injections currently are the two methods that most closely mimic normal insulin secretion. The ideal regimen for the newly diagnosed patient is a multiple dose, flexible basal‐bolus regimen that provides basal insulin throughout the day and night and insulin boluses before meals and snacks. Rapid‐acting insulin is injected approximately 15 minutes before eating; individual doses are adjusted meal‐to‐meal based on preprandial blood glucose values, anticipated meal macronutrient content, and physical activity. Practical considerations are vitally important when selecting an insulin regimen for a child with type 1 diabetes. Socio‐economic circumstances, parental health literacy and numeracy, patient’s age, supervision of care, ability and willingness to self‐administer insulin several times each day, and difficulty maintaining long‐term adherence, all conspire to make physiologic insulin replacement challenging. For these reasons, there is no universal insulin regimen that can be successfully used for all children with type 1 diabetes. The diabetes team must design an insulin regimen that meets the needs of the individual patient and is acceptable to the patient and family members(s) responsible for administering insulin to the child or supervising its administration. The route of insulin administration initially is determined by the severity of the child’s condition at presentation. Insulin is usually given intravenously for treatment of DKA; whereas insulin may be administered subcutaneously when children are metabolically stable without vomiting or significant dehydration and ketosis. Whenever appropriate, the newly diagnosed child should commence insulin replacement therapy with a flexible basal‐bolus regimen. In some healthcare systems, it is now possible to start insulin pump therapy at the time of diagnosis regardless of the severity of presentation or age of the child. Three major categories of insulin preparations, classified according to their time course of action, are available (Table 1.5) and various insulin replacement regimens consisting of a mixture of short‐ or rapid‐acting insulin and intermediate‐ or long‐acting insulin are used in children and adolescents, typically given at least two to four (or more) times daily. Table 1.5 Types of insulin preparations and approximate insulin action profiles. PA, protamine‐crystallized insulin aspart suspension; NPL, neutral protamine lispro suspension. PA + soluble aspart and NPL + lispro are both stable pre‐mixed combinations of intermediate‐ and rapid‐acting insulins. The human insulins and insulin analogues are available in vials, pre‐filled disposable pen injectors, and cartridges for non‐disposable pen injectors. These data are approximations from studies in adult test subjects. The times of onset, peak, and effective duration of action vary within and between patients and are affected by numerous factors, including size of dose, site and depth of injection, dilution, exercise, temperature, regional blood flow, local tissue reactions. a Dose‐dependent; 12 hours for 0.2 U/kg; 20–24 hours for ≥0.4 U/kg. Rapid‐acting insulin analogues incorporate amino acid substitutions, which make them quickly dissociate into monomers following injection and are then rapidly absorbed. Compared with short‐acting regular insulin, they produce lower postprandial glucose excursions. The three long‐acting insulin analogues are characterized by a relatively consistent and prolonged release of insulin without distinct peaks. Insulin glargine has a prolonged duration of action (22–24 hours) and can be injected at any time of day, but is usually given with dinner or at bedtime. The duration of action of insulin detemir is partly dependent on the dose – small doses may last only 12 hours; therefore, it usually has to be injected twice daily. Insulin degludec is an ultra‐long‐acting insulin with a flat, stable profile at steady state and a duration of action exceeding 24 hours and up to 42 hours. There is some evidence that both glargine and detemir lead to a decrease in the incidence of hypoglycaemia including nocturnal hypoglycaemia. Table 1.6 shows suggested starting total daily insulin dose (units per kg per day). Table 1.6 Suggested starting total daily insulin dose (units per kg per day). While clinical trials comparable to the Diabetes Control and Complications Trial (DCCT) have not been conducted in prepubertal children, it is logical to extrapolate that prepubertal children will also benefit from near‐normal glycaemic control. Intensive treatment regimens (MDI injections or insulin pump) are the preferred form of therapy for all patients with type 1 diabetes. Insulin regimens based on one or two daily injections cannot achieve optimal glycaemic control in type 1 diabetes except during the remission (‘honeymoon’) period, and may incur a greater risk of hypoglycaemia. These regimens, including the use of pre‐mixed combination insulins, should only be used when insurmountable barriers preclude the use of a multiple dose insulin regimen. When a two‐dose regimen is used, the total daily dose (TDD) is typically divided as follows: two‐thirds before breakfast and one‐third is given in the evening. The relative proportion of rapid‐ or short‐acting insulin to intermediate‐acting insulin depends on the pre‐meal blood glucose value and the carbohydrate content of meals. It is common to start by giving one‐third of the pre‐breakfast dose as rapid‐ or short‐acting insulin and two‐thirds as neutral protamine Hagedorn (NPH), and a similar ratio before dinner. For example, if the TDD for a 30‐kg child is 0.75 unit kg (22.5 units): a mixed dose injected before breakfast would consist of 5 units of rapid‐acting and 10 units NPH; the pre‐dinner dose would be 2.5 units rapid‐acting and 5 units NPH. Regular insulin should be injected at least 30 minutes before eating; rapid‐acting insulin (lispro, aspart, glulisine) is given 15 minutes before eating. The optimal ratio of rapid‐ or short‐acting to intermediate‐ or long‐acting insulin for each patient is determined empirically, guided by the results of frequent blood glucose monitoring. At least five daily blood glucose measurements are required to determine the effects of each component of the insulin regimen: before each meal, before the bedtime snack, and once overnight between midnight and 4 a.m. Parents are taught to look for patterns of hyperglycaemia or hypoglycaemia that indicate the need for a dose adjustment. Adjustments are made to individual components of the insulin regimen, usually in 5–10% increments or decrements, in response to patterns of consistently elevated (above the defined target range for several consecutive days) or unexplained low blood glucose levels, respectively. The insulin dose is adjusted until satisfactory blood glucose control is achieved, i.e. at least 50% of blood glucose values are in or close to the child’s target range. At the time of diagnosis, most children have some residual beta‐cell function and within several days to a few weeks often enter a period of partial remission (‘the honeymoon period’), during which normal or nearly normal glycaemia is achieved with a low dose of insulin. At this stage, the dose of insulin must be reduced to prevent hypoglycaemia, but should not be discontinued. As destruction of the remaining beta‐cells occurs over time, the insulin requirement increases (‘the intensification phase’), eventually reaching a full replacement dose. The average daily insulin dose in prepubertal children with long‐standing diabetes is approximately 0.6–0.8 units/kg/day, and in adolescents 1–1.2 units/kg/day. Obese patients usually are insulin‐resistant and require relatively higher TDDs, e.g. overweight or obese adolescents may require up to 1.5 units/kg/day. Beyond the remission period it is seldom possible without a regimented lifestyle and rigid adherence to a meal plan to achieve near‐normal glycaemia with two injections per day and without incurring a greater risk of hypoglycaemia, especially overnight. An important limitation of a two dose ‘split‐mixed’ regimen is that the peak effect of the pre‐dinner intermediate‐acting insulin (isophane, NPH) tends to occur at the time of lowest insulin requirement (i.e. from midnight to 4 a.m.), increasing the risk of nocturnal hypoglycaemia (Figure 1.2). Thereafter, insulin action decreases from 4 a.m. to 8 a.m., when basal insulin requirements normally increase. Consequently, the tendency for blood glucose levels to rise before breakfast (‘the dawn phenomenon’) is compounded by the waning insulin effect before breakfast and/or by counter‐regulatory hormones secreted in response to a fall in blood glucose levels during sleep, resulting in post‐hypoglycaemic hyperglycaemia (the Somogyi phenomenon, see Section 1.7.7.2). Figure 1.2 Schematic representations of commonly used insulin regimens. (a) Idealized insulin action profiles provided by a regimen consisting of twice‐daily mixtures of rapid‐acting insulin and intermediate‐acting insulin (NPH) injected before breakfast and before dinner. A snack must be eaten at bedtime to reduce risk of nocturnal hypoglycaemia. (b) Idealized insulin action profiles provided by a regimen consisting of a mixture of rapid‐acting insulin and intermediate‐acting insulin (NPH) before breakfast, rapid‐acting insulin before supper/dinner, and a second dose of NPH at bedtime, together with a snack to reduce risk of nocturnal hypoglycaemia. A three‐dose insulin regimen with mixed short‐ or rapid‐ and intermediate‐acting insulin before breakfast, only short‐ or rapid‐acting insulin before dinner, and intermediate‐ or long‐acting acting insulin at bedtime, may ameliorate these problems (Figure 1.2). The peak action of the morning dose of NPH occurring at midday may eliminate the need for a dose of rapid‐acting insulin at lunchtime (provided lunch does not contain excessive carbohydrate), and this may be necessary in circumstances where nobody is available to administer a pre‐lunch dose of rapid‐acting insulin to a young child. Insulin regimens that employ intermediate‐acting insulin demand consistency in the daily dietary regimen, both with respect to the amounts and timing of food consumed at each meal and snack, and the timing of insulin injections. Furthermore, owing to the time and duration of its peak action, NPH insulin given at bedtime is associated with increased frequency of nocturnal hypoglycaemia as compared to long‐acting, ‘peakless’ basal insulin analogues. Flexible basal‐bolus insulin regimens utilize MDI (Figure 1.3) or continuous subcutaneous insulin infusion (CSII) with an insulin pump (Figure 1.3). Flexible regimens more closely simulate normal diurnal insulin profiles, overcome many of the limitations inherent in split‐mixed regimens, and permit greater flexibility with respect to timing and content of meals, and adjustments for exercise. Doses of rapid‐acting insulin are selected before each meal or snack, based on pre‐meal glucose values, anticipated meal macronutrient content, and physical activity. In the ‘basal‐bolus’ MDI regimen (shown in Figure 1.3), a peakless, long‐acting insulin (insulin glargine, detemir, or degludec) is used to provide basal insulin (starting dose typically about 40–50% of the estimated TDD) together with short‐ or rapid‐acting insulin injected 30 or 15 minutes, respectively, before each meal. Figure 1.3 (a) Idealized insulin action provided by an insulin regimen consisting of four daily injections; rapid‐acting insulin before each meal (denoted by arrows) and a separate single daily injection of a long‐acting insulin analogue (glargine, detemir, or degludec), either at bedtime (as shown here) or at dinner or breakfast. (b) Idealized insulin effect provided by continuous subcutaneous insulin infusion via an insulin pump with rapid‐acting insulin. In this figure, alternative basal rates are illustrated; insulin delivery is programmed to decrease from midnight to 3 a.m. to decrease risk of nocturnal hypoglycaemia and to increase from 3 a.m. until 8 a.m. to combat the dawn phenomenon. Arrows indicate times of insulin injection or boluses before breakfast, lunch, and the evening meal (supper or dinner). Insulin glargine is usually administered once daily in the evening with dinner or at bedtime or before breakfast. It should be injected at about the same time each day; short‐ or rapid‐acting insulin is injected separately before each meal (and large snack), whenever it is eaten. Because it does not have the peak of activity characteristic of NPH, insulin glargine reduces the risk of nocturnal hypoglycaemia. Insulin detemir is an alternative long‐acting, ‘peakless’ basal insulin with pharmacodynamics characteristics similar to those of glargine especially during the first 12 hours after administration. It has a shorter duration of action than glargine and usually has to be administered twice daily in patients with severe insulin deficiency. Compared to NPH, studies in children and adolescents show a lower risk of hypoglycaemia and lower weight Z‐score while its effect on HbA1c is equivalent. The most recently developed long‐acting insulin formulation is ultralong‐acting insulin degludec, which forms multihexamers resulting in a depot after injection into the subcutaneous tissue. The hexamers slowly dissociate to form monomers that are rapidly absorbed into the circulation. Degludec has a flat and stable pharmacokinetic profile; its major advantage is that it does not have to be injected at precisely the same time each day, which is an attractive feature especially for adolescents whose lifestyles make it difficult to adhere to an inflexible schedule. Assuming the starting dose in a 60‐kg adolescent is 0.75 unit per kg or 45 units per day, the initial dose of long‐acting basal insulin might be 20 units (~45% of the TDD) and the insulin‐to‐carbohydrate ratio 1 unit of rapid‐acting insulin per 10 g carbohydrate (calculated using the formula 450/TDD). The initial correction or insulin sensitivity factor is calculated using the formula 1800/TDD. In this example, it would be 1 unit of rapid‐acting insulin to lower BG by 40 mg/dL (2.2 mmol/L) to a predetermined target; e.g. 120 mg/dL (11.1 mmol/L) during the day and 150 mg/dL (8.3 mmol/L) at bedtime and overnight. The actual prandial dose, administered ~15 minutes before the meal, is the sum of the individual doses required for carbohydrate coverage and the amount calculated to correct hyperglycaemia. The above simple formulae are useful to start insulin therapy; however, the optimal doses of rapid‐acting insulin must be determined empirically guided by the results of frequent BG measurements, before and ~2 hours after a meal. At least five daily measurements are required to determine the effects of each component of the insulin regimen. The BG concentration should be measured before each meal, at bedtime, and once between midnight and 4 a.m. Patients and parents are taught to look for patterns of hyperglycaemia (>180 mg/dL or 10 mmol/L) or hypoglycaemia (<70 mg/dL or 3.9 mmol/L) that indicate the need for a dose adjustment. Adjustments are made to individual components of the insulin regimen, usually in 5–10% increments or decrements, in response to patterns of consistently elevated (above the target range for several consecutive days) or unexplained low BG levels. The insulin dose is adjusted every 3–5 days until satisfactory BG control is achieved with at least 50% of BG values in the target range. Insulin has an effective shelf life of at least two years if refrigerated at 4 °C and can be kept at room temperature for up to one month. However, in tropical climates or if kept in a car interior on a hot day, insulin degrades more rapidly. Insulin is administered by a pen delivery system or with a syringe and needle, or via an insulin pump. In general, vials of insulin are cheaper than insulin in disposable pens or pen cartridges. For children with needle phobia, spring‐loaded automatic injection devices in which the needle is not visible may be helpful. Insulin may be administered using either a preloaded disposable pen or cartridges (different pens for rapid‐ and long‐acting insulins) for a reusable pen device. Pen delivery systems are generally preferred by children as they are quicker and easier to use than syringes and needles, and lead to greater independence. Insulin for injection may be drawn up from a vial and injected using an insulin syringe and needle. When mixing insulins, the rapid‐acting clear insulin should be drawn up into the syringe before the intermediate‐acting (NPH, isophane), which is a cloudy suspension, i.e. ‘clear before cloudy insulin’. Before drawing up the dose into a syringe or injecting via a pen device, any preparation containing intermediate‐acting insulin should be gently inverted several times to ensure that the insulin is uniformly suspended. Note that all insulin analogues are clear solutions; patients must be counselled to take special care not to confuse the rapid‐acting with the long‐acting analogue and accidentally (thinking it is the long‐acting insulin) inject a large dose of rapid‐acting insulin at bedtime. The age at which children start to give their own injections is variable. Peer pressure, which may be experienced by a child attending a diabetes camp where children may see their contemporaries or even younger children administering injections, may motivate a child to learn to self‐inject. Parents are strongly advised to supervise insulin injections when children wish to become more independent. Appropriate injection sites are shown in Figure 1.4. The use of different injection sites and rotation of these sites should be encouraged to avoid the development of lipohypertrophy (Figure 1.5) which may be unsightly and lead to erratic absorption of insulin. If patients avoid injecting into these areas, lipohypertrophy will gradually resolve over a period of several months. Figure 1.4 Insulin injection sites. Figure 1.5 Lipohypertrophy on the arm. When using short (e.g. 4 or 5 mm) needles, the injection is given at a 90° angle without pinching the skin unless the patient is very thin. In those who find injections painful, distraction techniques can be used or the skin can be numbed with an ice pack before the injection. In infants, the injection of doses as small as 0.5 to 1 unit can result in significant inaccuracies, with the dose actually delivered ranging from 0.89 to 1.23 units. In these circumstances, use of a pen‐delivery system, which allows injections of insulin in increments of 0.5 units is recommended. If possible, infants should be managed using an insulin pump which permits accurate delivery of tiny amounts of insulin. Alternatively, insulin may have to be diluted to U10 concentration: 1 line on an insulin syringe corresponds to 0.1 unit of insulin. In solution, human insulin forms hexamers (six‐molecule units). The rate of absorption after subcutaneous injection is principally determined by how quickly the hexamers dissociate into monomers (single molecules), which are absorbed across endothelial barriers. Children should be treated with human insulin analogues in a concentration of 100 units /ml (U100). Table 1.5 shows the most common insulin preparations and their durations of action. Despite the introduction of CSII (pump) therapy nearly 40 years ago, widespread adoption of this technology in paediatric diabetes practice has only occurred in the last 15 years. In 1996, less than 5% of patients who started pump therapy were < 20 years of age; over the intervening years there has been a worldwide steady increase in the number of children and adolescents using pump therapy. This may be attributable to recognition of the crucial importance of lowering HbA1c levels to prevent or delay vascular complications together with advances in pump technology. There is considerable worldwide variation in the frequency of pump use, e.g. use of insulin pumps is threefold greater in Germany, Austria, and the United States compared with England and Wales. An insulin pump has several advantages over insulin injections, e.g. the ability to programme changes in basal insulin delivery to meet an anticipated increase or decrease in need. This feature can be advantageous in combating the dawn phenomenon (especially pronounced in adolescents) or preventing hypoglycaemia during or after strenuous exercise. In addition to programming various basal infusion rates over the course of the day and night, the use of dual wave and square wave bolus delivery significantly lowers postprandial blood glucose levels after meals with a high fat and protein content. Also, because the infusion set typically only has to be replaced every two or three days, the child is spared the discomfort of numerous daily injections. Registries that track outcomes of type 1 diabetes treatment and several single centre observational studies show that children who use a pump have significantly lower HbA1c levels and decreased glycaemic variability, decreased rates of severe hypoglycaemia, and improvements in diabetes‐related quality of life, treatment satisfaction, and less fear of hypoglycaemia. A meta‐analysis of randomized controlled clinical trials showed that CSII results in a small (~0.5%) improvement in HbA1c (Garvey and Wolfsdorf 2015). Even larger improvements in glycaemic control and hypoglycaemia reduction are possible with insulin pumps that incorporate continuous glucose monitoring (CGM), referred to as sensor augmented pump therapy (Slover et al. 2012). Although an insulin pump is a complex and sophisticated medical device that requires extensive training in its proper use, with appropriate education and training and with support from parents and a school nurse, most children and adolescents can manage to successfully and safely use an insulin pump and benefit from its advantages. Because only short‐ or rapid‐acting insulin is used with CSII, any interruption in insulin delivery rapidly leads to metabolic decompensation. To reduce this risk, patients must pay meticulous attention to insuring the integrity of the infusion system and must measure their blood glucose levels at least four times daily. A recent study showed a lower rate of DKA with pumps as compared to MDI therapy. Success requires motivation to achieve normal blood glucose levels, frequent blood glucose monitoring, accurate carbohydrate counting, good record‐keeping, and frequent contact with the diabetes team. Patients must understand that to be successful CSII therapy requires more time, effort, and active involvement in diabetes care by patients and parents, and considerable education and support from the diabetes team. The individual who is unable to master an MDI regimen is not likely to be successful with CSII. Despite concerns that it might have adverse psychosocial consequences owing to the added burden of treatment, especially in adolescents, the opposite effect has been observed. The insulin delivery system consists of a programmable pump (about the size of a cell phone) containing a reservoir filled with rapid‐acting insulin, which is connected by a plastic infusion tube to a small plastic (or metal) cannula inserted subcutaneously ‐ usually in the abdomen or posterior aspect of the upper arm, anterior thigh or buttocks (in toddlers and infants it is most often inserted in the buttocks) and fixed in place by adhesive tape. Depending on the specific device, changes in the insulin infusion rate as small as 0.025 units /hour can be made. The cannula is usually left in place for two to three days. If the cannula is not changed regularly or if the same site is used recurrently, there is a risk of developing lipohypertrophy or developing an infection at the site. A 24‐hour profile including measurements before each meal, two hours after the meal, at midnight, at 2.00–4.00 a.m. should be carried out at least every other week to enable any necessary dose changes to be made. The absence of a long‐acting insulin depot means that if there is an interruption in insulin delivery, blood glucose levels will rise quickly and ketosis can develop in four to six hours. CSII therapy is considerably more expensive than MDI therapy. Some patients prefer a tubeless disposable ‘patch pump’ (Figure 1.6), consisting of a micro pump, an insulin reservoir, and a cannula; the integrated device attaches directly to the skin. Insulin is delivered through a small subcutaneous catheter. Patch pumps are free of tubing and are waterproof, which makes them more discreet and allows greater freedom with activities such as swimming. They are disposable and are lighter and smaller than conventional pumps. They usually need to be reapplied every two or three days. The pump is controlled by a remote device that has an integrated glucose meter and which communicates wirelessly with the patch pump. Figure 1.6 An Omnipod® patch pump is on the left buttock and a Dexcom G5® glucose sensor on the right buttock. CSII can also be used together with CGM sensors that measure glucose levels in the interstitial fluid every few minutes, referred to as sensor augmented pump therapy. The data can be transferred to the pump using Bluetooth technology so that if the blood glucose is below a certain value, for example, 4 mmol/L (72 mg/dL), the insulin pump sets off an alarm and suspends insulin delivery. Some insulin pumps can also be set to raise an alarm if the glucose level is falling rapidly and they will suspend insulin delivery if hypoglycaemia is anticipated to occur within the next 30 minutes (‘predictive low glucose suspend’). Recently, interstitial glucose sensing and insulin delivery have been combined to produce a ‘hybrid closed loop system’, i.e. a version of an artificial pancreas that has been approved for use from 7 years. Such systems increase the number of BG values in the target range and decrease hypoglycaemia. Algorithms to cope with situations such as exercise are currently being devised and new technological innovations and device improvements have had a beneficial impact on the management of diabetes in children and adolescents. Use of an insulin pump permits a flexible lifestyle and eating patterns and is associated with a high degree of satisfaction in appropriately motivated patients. The technological innovations described above have provided patients with insulin preparations whose pharmacokinetic properties make it possible to crudely simulate physiologic insulin kinetics. It is now possible for children to safely achieve unprecedented levels of glycaemic control without excessive severe hypoglycaemia, and yet in most patients the goal of near‐normalization of glycaemic control is not realized. Several recent studies have shown a persistent gap between target HbA1c ranges and the actual values patients attain. The successful implementation of intensive diabetes therapy remains a major challenge, and diabetes care providers should frankly discuss treatment options with the child and parents and explain the advantages and disadvantages of each in attempting to meet the overall goals of treatment. The most suitable regimen for a given child and family should be determined by mutual consent and should be the regimen they will most likely be able to afford and adhere to. These considerations are particularly important in managing children living in economically and socially disadvantaged circumstances and when there is a shortage of food. The dawn phenomenon describes the rise in insulin requirements and blood glucose concentrations in the early morning, approximately 5.00–8.00 a.m. It is most pronounced during puberty and is thought to be caused primarily by the insulin resistance produced by nocturnal growth hormone secretion. This is a difficult problem to resolve in those using twice daily insulin regimens. Possible benefit may be obtained in such patients by dividing the evening injection so that rapid‐acting insulin is given prior to the evening meal and intermediate‐acting insulin before bedtime (see Figure 1.2a). Alternatively, in patients on a basal‐bolus regimen, the pre‐bedtime dose of intermediate‐acting insulin or long‐acting insulin analogue can be increased. When using an insulin pump, the overnight basal rate can be programmed to increase at 3 a.m. until 8 a.m. This term refers to morning hyperglycaemia caused by the release of counter‐regulatory hormones following the occurrence of nocturnal hypoglycaemia. However, when pronounced hyperglycaemia occurs after an episode of nocturnal hypoglycaemia, it usually is attributable to ingestion of an excessive amount of carbohydrate to treat the hypoglycaemia episode. Alternatively, when pre‐breakfast hyperglycaemia occurs without preceding nocturnal hypoglycaemia, this usually is caused by inadequate or waning overnight insulinemia. Caring for young children with diabetes is challenging for many reasons; one is the need to accurately and reproducibly measure and inject tiny doses of insulin supplied in a concentration of 100 units/ml (U100 insulin). To administer a dose of 1 unit requires the ability to accurately measure 10 μl (1/100 ml) of insulin. A dose change of 0.25 U translates into a volume difference of 2.5 μl in a 300 μl (3/10 cc or 30 unit) syringe. When parents attempt to measure insulin doses in increments of 0.25 U (e.g. 3.0, 3.25, 3.5 U) using a standard commercial 30 unit (300 μl) syringe, they consistently measure more than the prescribed amount. For these reasons, CSII can be a useful tool to provide U100 insulin in small doses to young children when there is appropriate caregiver education and diabetes team support. However, when injection regimens are used, to enhance accuracy and reproducibility of small doses, insulin should be diluted to U10 (10 units/ml) with the specific diluent available from the insulin manufacturers. Using U10 insulin, each line (‘unit’) on a syringe is actually 0.1 U of insulin. Nutrition is a cornerstone of diabetes management and nutrition education and counselling are essential components of a comprehensive programme of diabetes self‐management education for patients and their families. There is no ‘diabetic diet’ per se; rather, nutrition therapy should be individualized according to the family’s and patient’s usual eating habits and food preferences, religious or cultural considerations, and access to food. Combining blood glucose monitoring with intensive insulin therapy and mastery of carbohydrate counting enables children and adolescents to enjoy dietary flexibility while maintaining glycaemic control in the target range. The focus of dietary management differs between the two major types of diabetes. In type 1 diabetes, the primary goal is to match insulin delivery and carbohydrate consumption to achieve blood glucose levels in the target range. In contrast, children with type 2 diabetes are typically overweight or obese at presentation so that the emphasis is on weight loss, limiting calorie intake, and distributing meals evenly throughout the day. Growth is an excellent indicator of the adequacy of energy intake, and should be regularly evaluated by plotting height and weight on a growth chart. If growth is not optimal, the diet should be reviewed and, in patients with type 1 diabetes, one should consider inadequate insulin delivery, hypo‐ or hyperthyroidism, coeliac disease, and adrenal insufficiency as possible causes. For overweight (BMI ≥85th percentile) and obese (BMI ≥95th percentile) youth with either type 1 diabetes or type 2 diabetes, energy consumption must be reduced to arrest weight gain. Table 1.7 shows a general approach to meal management for both type 1 diabetes and type 2 diabetes. Table 1.7 General approaches to meal management. The numerous commercially available ‘diabetic foods’ are not recommended for children with diabetes, as such foods tend to be expensive and have no particular advantages over a healthy diet based on normal foods. Some ‘diabetic foods’ also contain the sweetener sorbitol that may lead to diarrhoea when consumed in large amounts. ‘Diabetic foods’ may also have a high calorie and fat content. Drinks containing sugar should be replaced with those containing artificial sweeteners. Carbohydrate is the main nutrient in starches, fruits, milk, and sugar‐containing foods and is the major determinant of postprandial blood glucose excursions. The most widely used method for youth with type 1 diabetes who use rapid‐acting prandial insulin is an individualized insulin‐to‐carbohydrate ratio (e.g. 1 unit per x grams of carbohydrate). Carbohydrate counting allows flexibility in food choices and enables patients to include a wide variety of foods in their meal plan. An important limitation of carbohydrate counting is that it does not address carbohydrate quality, diet composition, or total caloric intake. Patients should be encouraged to learn about the glycaemic index (GI) of their favourite carbohydrate‐containing foods (‘fast’ vs. ‘slow’ carbohydrates). Carbohydrates are absorbed slowly from low‐GI foods, whereas high‐GI foods lead to rapid carbohydrate absorption and a brisk increase in blood glucose levels. The amount of fat, protein, and fibre in food also influences the rate of carbohydrate absorption. For example, meals with a high fat content slow the rate of carbohydrate absorption. An alternative method, employed by patients who use a fixed dose insulin regimen or for whom carbohydrate counting may be too difficult, is a prescribed carbohydrate meal plan that maintains day‐to‐day consistency in the carbohydrate content of meals and snacks. Children and their parents are taught how to read the nutrition facts on food labels for total carbohydrate (grams) per serving. Periodically measuring and weighing foods is recommended to reinforce accurate portion sizes and carbohydrate amounts, which is essential for optimal insulin dosing and to minimize postprandial hyperglycaemia. Numerous nutrition ‘apps’ are available for smart phones and other digital devices. Renewed attention has been given to the impact of fat and protein on postprandial glycaemic responses in youth with type 1 diabetes, and evidence suggests that additional insulin to account for protein and fat in a meal is superior to dosing insulin solely based on carbohydrate intake. Diabetes is associated with a high risk of early subclinical and clinical cardiovascular disease (CVD), and children with type 1 diabetes are in the highest tier for cardiovascular risk. Saturated and trans fatty acids are the principal dietary determinants of plasma low‐density lipoprotein (LDL)‐cholesterol concentrations. To reduce the risk of CVD, consumption of saturated fat, trans fatty acids, and cholesterol must be limited while increasing intake of unsaturated fats and omega‐3 fatty acids. Because both glycaemic control and cardioprotective nutrition improve the lipid profile and reduce cardiac risk, patients should be advised to consume less red meat and high‐fat dairy foods and eat more poultry, fish, and vegetable proteins, and drink low‐fat milk. Children and adolescents with normal plasma lipid concentrations should derive less than 10% of their energy from saturated fats; the daily intake of cholesterol should be <300 mg per day, and consumption of trans fatty acids (principally formed by the hydrogenation of unsaturated fats and present in margarine, deep‐frying fat, and some processed foods such as biscuits and cakes) should be minimal. In the overweight or obese child, total fat consumption should be reduced. Newly diagnosed children with type 1 diabetes usually present with weight loss; therefore, the initial meal plan includes an estimation of energy requirements to restore and then maintain an appropriate body weight and allow for normal growth and development. Energy requirements vary with age, height, weight, stage of puberty, and level of physical activity. Because the energy needs of growing children continuously change, the meal plan should be re‐evaluated at least every six months in young children and annually in adolescents. Dietary management begins at the time of diagnosis with an assessment by a registered dietitian (clinical nutritionist). The meal plan must take account of the child’s school schedule, early or late lunches, physical education classes, after‐school physical activity, and differences in a child’s activities on weekdays compared with weekends and holidays. Young children typically have three meals and two or three snacks daily, depending on the interval of time between meals, age of the child, and level of physical activity. Although their daily energy intake is relatively constant over time, young children adjust their energy intake at successive meals. The highly variable food consumption from meal‐to‐meal typical of normal young children is especially challenging when the child has type 1 diabetes. The purpose of snacks is to prevent hypoglycaemia and hunger between meals. If the basal insulin component is adjusted appropriately, patients who use a basal‐bolus insulin regimen or pump therapy may not require snacks. The dietitian’s role is to evaluate the patient’s and family’s knowledge and understanding of nutrition and to formulate an individualized meal plan. Nutrition education and counselling, like all aspects of diabetes education, should be an ongoing process with periodic review and revision of the meal plan and assessment of the child’s and parents’ levels of comprehension, ability to analyse and solve problems, and adherence to the nutrition goals. The patient should return to see the dietitian if glycaemic control is poor, growth is failing, weight gain is excessive, or if other problems arise related to dietary management. The epidemic of obesity has not spared children with type 1 diabetes, and a recent report from the SEARCH study in the US showed a higher prevalence of overweight in youth with type 1 diabetes compared with similarly aged youth without diabetes (22% vs. 16%) (Liu et al. 2010). There is wide geographic variation in the frequency of DKA at presentation of diabetes; worldwide incidence rates range from approximately 15–80%. DKA is a presenting feature in 6–11% of patients with type 2 diabetes. In children with established diabetes, the risk of DKA is increased in those with chronic poor metabolic control and previous episodes of DKA, adolescent girls, children with psychiatric disorders, and those with psychosocial challenges. In patients who use a pump, interruption of insulin delivery for any reason can lead to DKA. Most DKA episodes are caused by insulin omission or treatment error, and the majority of the remainder are due to inadequate insulin treatment during an intercurrent illness. The diagnostic criteria for the diagnosis of DKA include: hyperglycaemia (glucose>11 mmol/L [200 mg/dL]) with a venous pH < 7.30 or serum bicarbonate <15 mmol/L. Blood BOHB is typically ≥3.0 mmol/L and ketonuria is ‘moderate or large’. DKA is classified by its severity: mild (venous pH < 7.30 or bicarbonate<15 mmol/)L, moderate (pH < 7.2 or bicarbonate<10 mmol/L) and severe (pH < 7.1 or bicarbonate <5 mmol/L). Partially treated children and children who have consumed little or no carbohydrate may have only modestly elevated blood glucose concentrations, referred to as ‘euglycaemic ketoacidosis’. The mortality rate from DKA is approximately 0.2%. Death is usually caused by cerebral oedema, but may also be caused by hypokalaemia‐induced cardiac arrhythmia, sepsis, aspiration pneumonia, and numerous other rare complications. Acute management should follow the general guidelines for paediatric advanced life support (PALS). The protocol described below for the treatment of DKA is largely based on the guidelines published by the International Society of Pediatric and Adolescent Diabetes (Wolfsdorf et al. 2018). If the child is too ill to weigh, for the purposes of calculating fluid requirements, weight can be estimated from a recent clinic weight or from a centile chart. Antibiotics should be given if sepsis is thought likely after appropriate samples for culture have been taken. Whenever possible, the child with DKA should be cared for in a high dependency or intensive care unit with experienced nursing staff, and with access to a clinical chemistry laboratory that can provide timely measurements of serum chemistries. In hospitals without a high dependency unit, high dependency care can still be given by providing a high level of nursing care, often on a one‐to‐one basis. Children with severe DKA – pH <7.1 or bicarbonate <5 mmol/L, compromised circulation, depressed level of consciousness, and those at increased risk for cerebral oedema (<5 years of age) should be treated in a paediatric intensive care unit or in a children’s ward that specializes in diabetes and can provide comparable resources and supervision of care. The following should be documented: The objectives of fluid and electrolyte replacement therapy are: (i) to restore circulating volume; (ii) to replace sodium, potassium, and the extracellular and intracellular water deficits; and (iii) to improve glomerular filtration and thereby enhance the clearance of glucose and ketones from the blood Patients with DKA have a deficit in extracellular fluid (ECF) volume usually in the range 3–10%. Clinical estimates of the volume deficit are subjective and inaccurate. For fluid calculations, use 3–5% in mild DKA, 5–7% in moderate DKA, and 7–10% in cases of severe DKA. For patients with severe volume depletion but not in shock, volume expansion (resuscitation) should begin immediately with 0.9% saline to restore the peripheral circulation. The volume administered typically is 10–20 ml/kg over 1–2 hours, and may need to be repeated until perfusion is adequate. In the rare patient with DKA in shock, rapidly restore circulatory volume with isotonic saline in 20 ml/kg boluses infused as quickly as possible through a large bore cannula with reassessment after each bolus. Subsequent fluid management (deficit replacement) can be accomplished with 0.45–0.9% saline or a balanced salt solution such as Ringer’s lactate, Hartmann’s solution, or Plasmalyte. Fluid therapy should begin with deficit replacement plus maintenance fluid requirements. All children will experience a decrease in vascular volume when plasma glucose concentrations fall during treatment. Therefore, it is essential to ensure that they receive sufficient fluid and salt to maintain adequate tissue perfusion. Deficit replacement should be with a solution that has a tonicity equal to or greater than 0.45% saline (with added potassium chloride, phosphate or potassium acetate; see Section 1.9.5). The decision whether to use an isotonic or a hypotonic solution for deficit replacement should depend on clinical judgement based on the patient’s hydration status, the serum sodium concentration, and the effective osmolality. In addition to providing the usual daily maintenance fluid requirement (see example in Table 1.9), replace the estimated fluid deficit at an even rate over 36–48 hours. Except for severely ill patients, oral intake typically begins within 24 hours. At this point, any remaining deficits are replenished by oral intake once DKA has resolved and patients are transitioned to subcutaneous insulin. Clinical assessment of hydration status and calculated effective osmolality are valuable guides to fluid and electrolyte therapy. The aim is gradually to reduce serum effective osmolality to normal. There should be a concomitant increase in serum sodium concentration as the glucose concentration decreases (sodium should rise by 0.5 mmol/L for each 1 mmol/L decrease in glucose concentration). Urinary losses should not routinely be added to the calculation of replacement fluid but this may be necessary in rare circumstances such as a patient with severe dehydration and extreme hyperglycaemia (i.e. mixed DKA and hyperglycaemic hyperosmolar state [HHS]). The sodium content of the fluid should be increased if measured serum sodium concentration is low and does not rise appropriately as the plasma glucose concentration falls. The fluid infused during initial resuscitation to restore the circulation should be taken into account when calculating fluid requirements and deducted from the total. Maintenance fluid requirements can be estimated using the Holliday‐Segar method in Table 1.8 or the body surface area method, 1500 ml/m2/24 hour (not suitable for use in children <10 kg). Using the Holliday‐Segar method to determine the maintenance fluid requirement for an 8‐year‐old child weighing 25 kg yields 65 ml per hour or 1600 ml per day. This method is not suitable for neonates <14 days old and generally overestimates fluid needs in neonates. Table 1.8 Maintenance fluid requirements in DKA. The hourly infusion rate is calculated using the following formula:
Diabetes Mellitus
Definition
or
or
or
Indications
Confirmation of the diagnosis of diabetes mellitus in uncertain cases and diagnosis of impaired glucose tolerance
Preparation
Perform in the morning after fasting overnight for at least eight hours
Procedure
Interpretation
Incidence
Type 1 diabetes caused by autoimmune‐mediated ß‐cell destruction (and idiopathic forms of β‐cell dysfunction) usually leading to severe or absolute insulin deficiency
Type 2 diabetes caused by progressive loss of insulin secretion on a background of insulin resistance.
Monogenic diabetes syndromes such as neonatal diabetes and maturity‐onset diabetes of the young (MODY)
Diseases of the exocrine pancreas (such as cystic fibrosis)
Drug‐ or chemical‐induced diabetes such as with glucocorticoid use, drugs used for treatment of HIV/AIDS, or after organ transplantation
Gestational diabetes mellitus diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation
Environment
Vaccines
Idiopathic type 1 diabetes
Biochemistry
Clinical presentation
History
Examination
Differential diagnosis
Investigations
Initial management of newly diagnosed type 1 diabetes
Management of the child presenting without ketoacidosis
Hospitalization vs. Outpatient (home) treatment
Outpatient diabetes care
The diabetes team
Initial diabetes education
Main topics for discussion following diagnosis
Requirements on discharge from hospital
Lancing device and lancets
Blood glucose meter and test strips
Blood ketone meter and ketone strips or urine ketone test strips
Oral glucose tablets and gel
Glucagon emergency kit
Sharps container
Literature on diabetes management and how to obtain a Medic Alert bracelet/necklace
Pen insulin delivery system, disposable pre‐filled pens, or syringes with needles for insulin injections
Insulin cartridges for non‐disposable pen‐delivery system or insulin vials
Rapid‐acting and long‐ or intermediate‐acting insulin (depending on insulin regimen)
Alcohol swabs
Needle clipper
Continuing diabetes education and long‐term supervision of diabetes care
Psychosocial aspects of diabetes management
Diabetes Control and Complications Trial (DCCT)
Goals of therapy
Insulin therapy in type 1 diabetes
Initial insulin therapy
Insulin type
Onset of action (h)
Peak of action (h)
Effective duration of action (h)
Rapid‐acting analogues
Aspart, lispro, glulisine
0.25–0.5
1–3
3–5
Regular insulin
0.5–1
2–4
5–8
Intermediate‐acting insulin
Neutral Protamine Hagedorn (NPH), isophane
2–4
4–10
10–16
Long‐acting analogues
Detemir
Glargine
Degludec
2–4
2–4
2–4
None
None
None
12–20a
20–24
24–42
Premixed combinations
50% NPH, 50% regular
50% NPL, 50% lispro
70% NPH, 30% regular
70% PA, 30% aspart
75% NPL, 25% lispro
0.5–1
0.25
0.5–1
0.25
0.25
dual
dual
dual
dual
dual
10–16
10–16
10–16
15–18
10–16
Insulin analogues
No DKA at presentation
DKA at presentation
<6 years or HbA1c <7%
0.15–0.25
0.5–0.75
Prepuberty
0.25–0.5
0.75–1
Puberty
0.5–0.75
1–1.2
Postpuberty
0.25–0.5
0.75–1
Split‐mixed insulin regimens
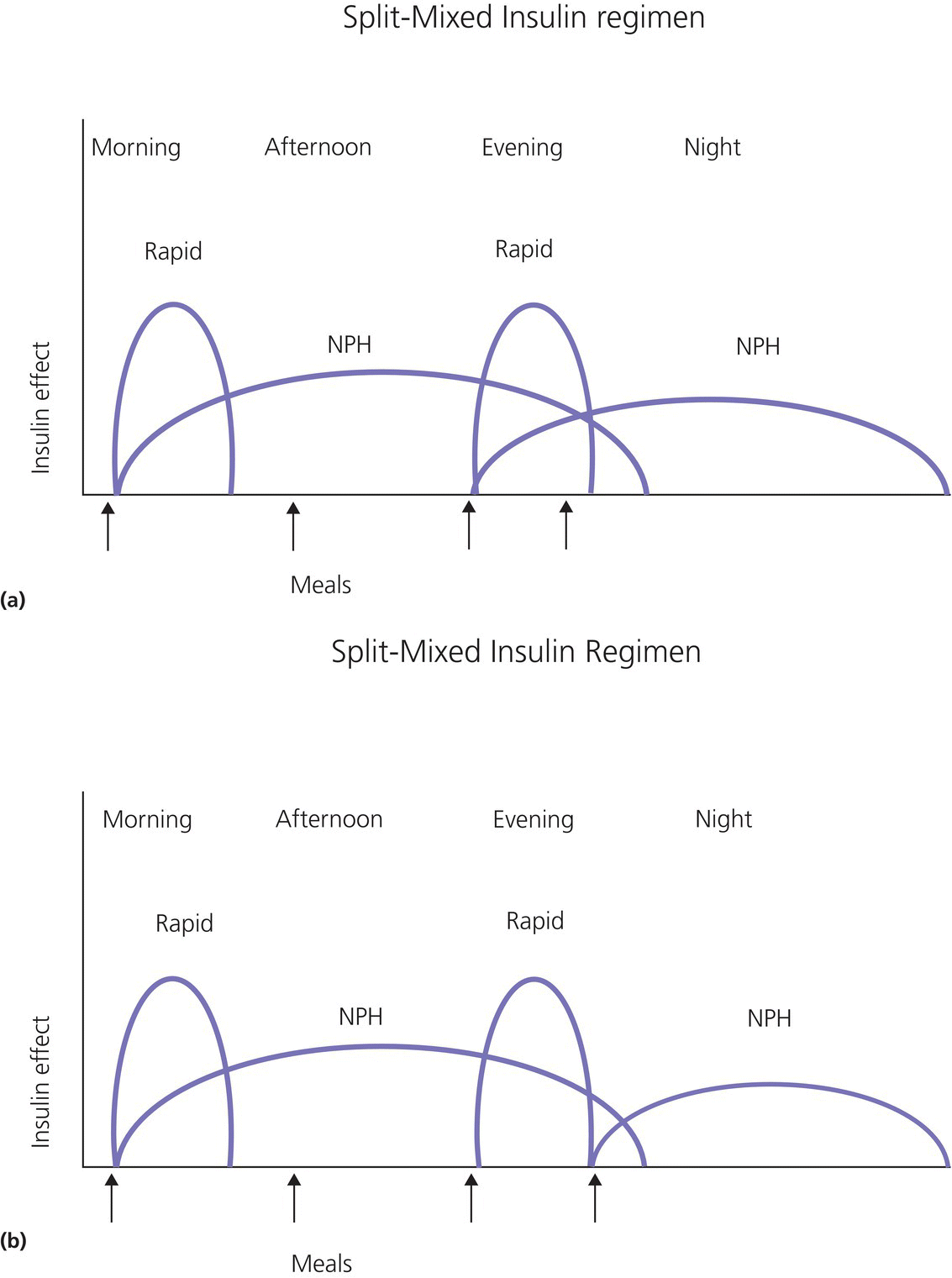
Basal‐bolus regimens and continuous subcutaneous insulin infusion (CSII)
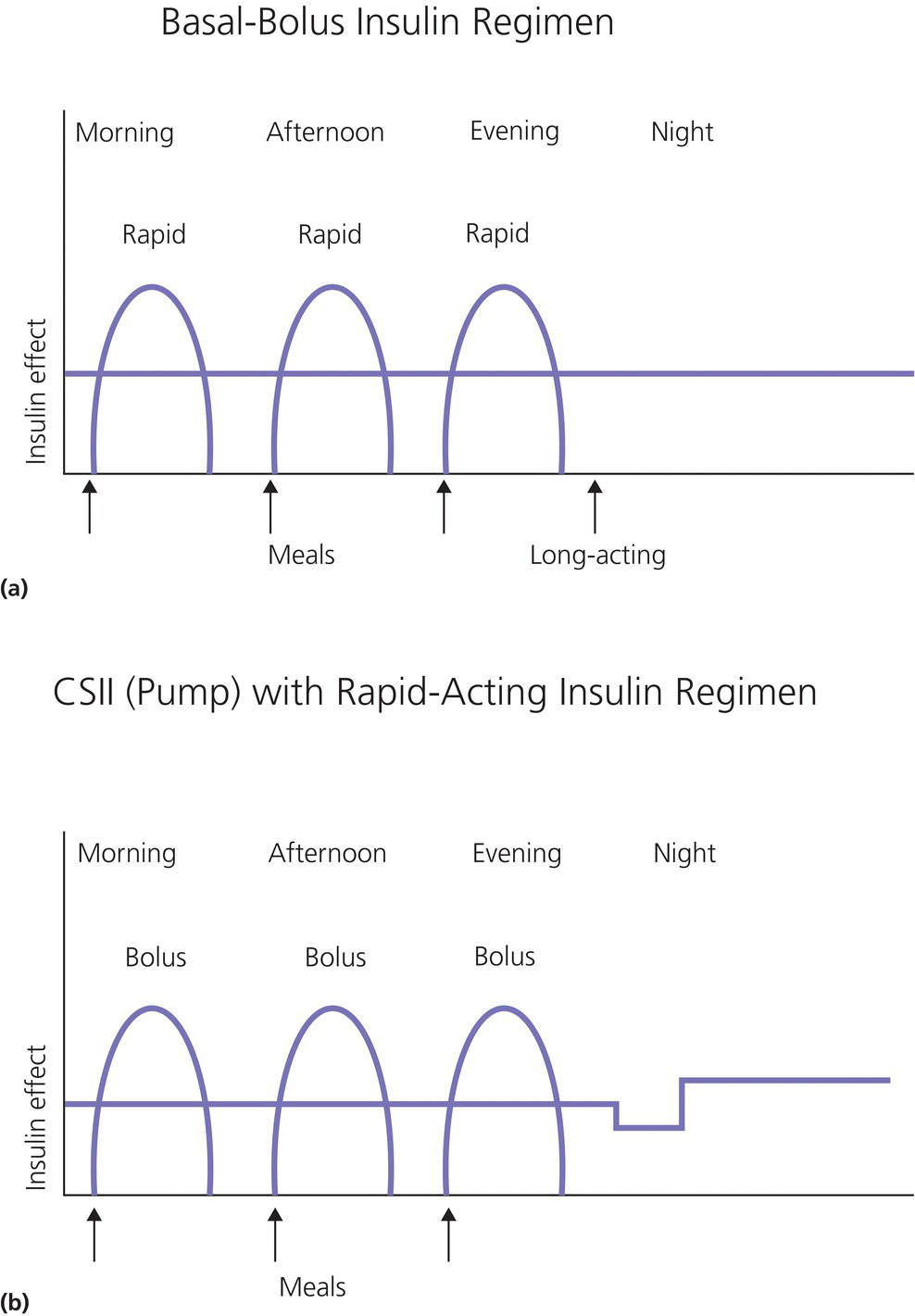
Practical aspects of insulin treatment
Insulin delivery systems
Insulin pens
Syringes and needles
Injections
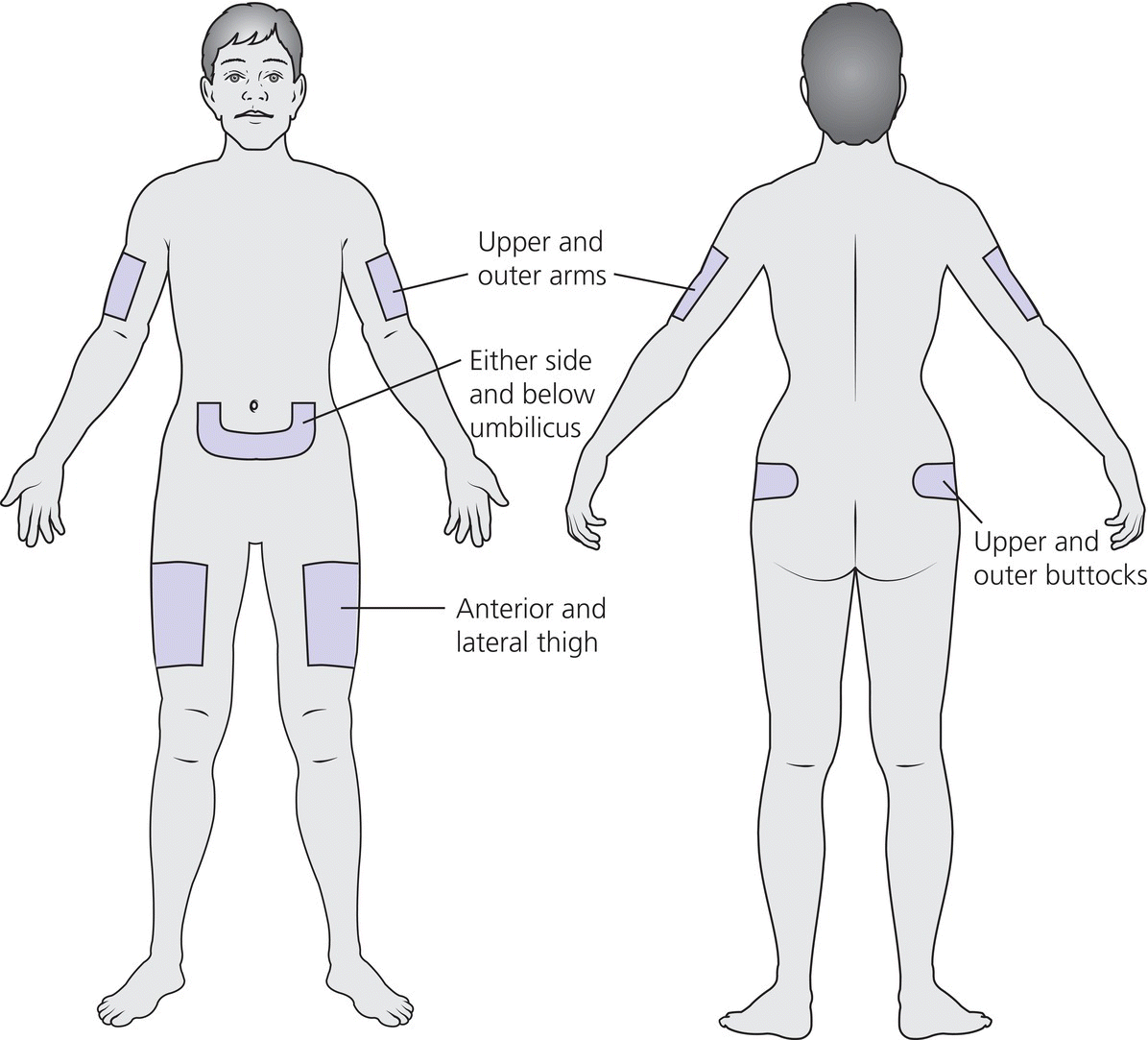
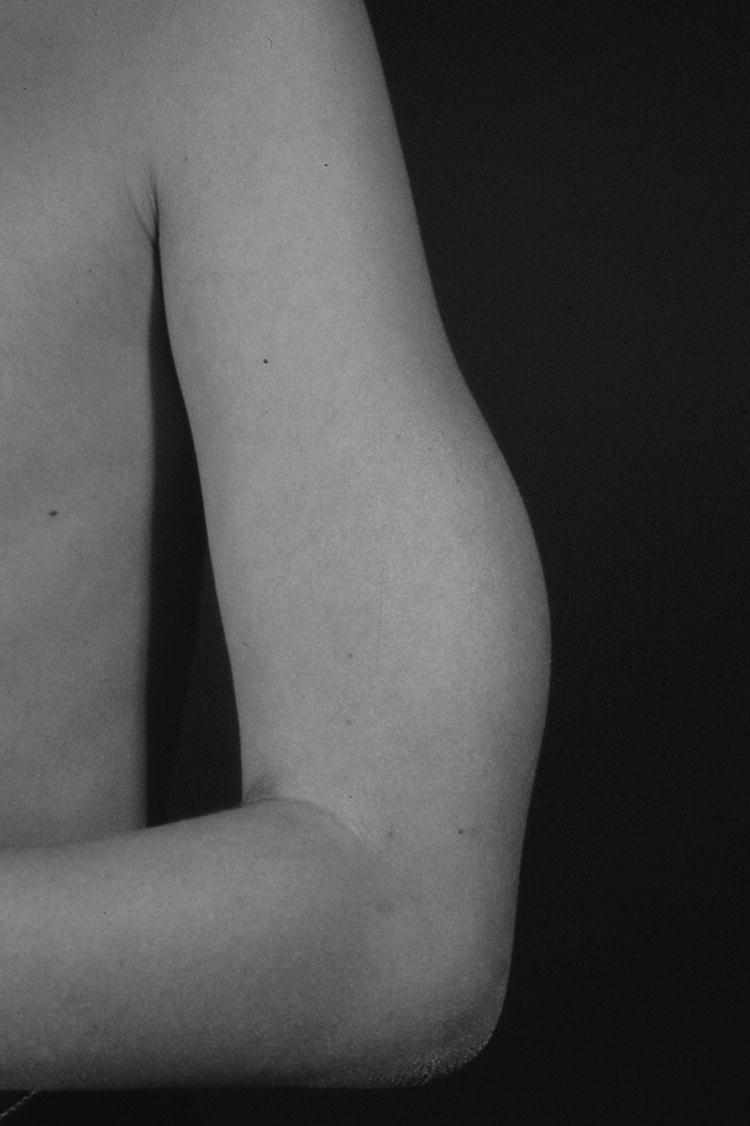
Injection technique
Injecting small doses
Insulin preparations
Continuous subcutaneous insulin infusion (insulin pumps)
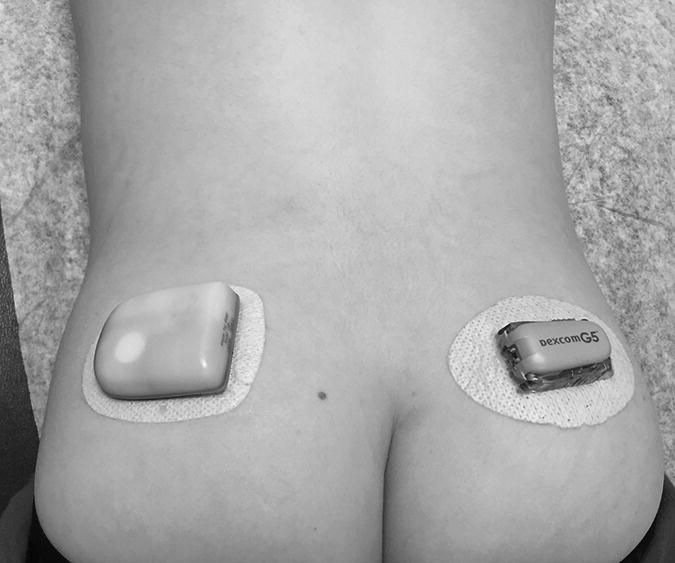
Insulin regimens and glycaemic control
Potential problems with insulin therapy
The dawn phenomenon
The Somogyi phenomenon
Insulin therapy in young children: special considerations
Dietary management
Type 1 diabetes
Split‐mixed insulin regimen
Flexible basal‐bolus or insulin pump therapy
Type 2 diabetes
Carbohydrate counting
Fat
Nutrition education and formulation of the meal plan
Management of the child presenting with ketoacidosis
Resuscitation
Initial monitoring
Fluid therapy
Fluid requirements
Weight
Maintenance fluid requirements (ml per kg per hour)
Maintenance fluid requirements (ml per kg per day)
First 10 kg
4 ml/kg × 10 kg = 40 ml/hr
100 ml/kg × 10 kg = 1,000 ml/d
Second 10 kg
2 ml/kg × 10 kg = 20 ml/hr
50 ml/kg × 10 kg = 500 ml/d
Each additional 1 kg
1 ml/kg × 5 kg = 5 ml/hr
20 ml/kg × 5 kg = 100 ml/d
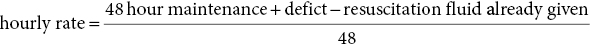
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree