Fig. 5.1
The growth of gross domestic product (GDP) and prevalence of diabetes increased in a parallel manner in China during the last three decades [3]
At a glance | 2013 | 2035 |
|---|---|---|
Total population (millions) | 2278 | 2476 |
Adult population (20–79 years, millions) | 1613 | 1818 |
Diabetes (20–79 years) | ||
Regional prevalence (%) | 8.6 | 11.1 |
Comparative prevalence (%)* | 8.1 | 8.4 |
Number of people with diabetes (millions) | 138.2 | 201.8 |
IGT (20–79 years) | ||
Regional prevalence (%) | 6.8 | 9.0 |
Comparative prevalence (%)* | 6.6 | 7.8 |
Number of people with IGT (millions) | 110.1 | 164.5 |
Type 1 diabetes (0–14 years) | ||
Number of children with type 1 diabetes (thousands) | 32.5 | – |
Number of newly diagnosed cases per year (thousands) | 5.3 | – |
Health expenditure due to diabetes (20–79 years, USD) | ||
Total health expenditure, R = 2#, (billions) | 88.4 | 98.4 |
Adding to this health-care challenge are the rapid societal transition and changing demographics occurring within an unprepared health-care system designed for provision for acute and episodic rather than chronic care. In many emerging economies, the lack of societal, environmental and financial protection has led to the widening social disparity which has exposed the genetically vulnerable and socially disadvantaged to develop this silent killer often due to lack of information, awareness and intervention [2, 3]. In this chapter, we will review the epidemiology, aetiology, morbidity, diagnosis, treatment and care delivery for diabetes focusing in China and WP Region. We will also discuss the current unmet needs and possible solutions for the prevention and control of diabetes in this populous region.
Rapid Transition and the Obesity Epidemic
The impact of rapid urbanisation on the diabetes epidemic is exemplified by the parallel increase in gross domestic product (GDP) and diabetes prevalence in China (Fig. 5.1). In less than three decades, while the GDP has increased by 100-fold, the prevalence of diabetes has also increased by tenfold. In the 2010 National Survey of 98,658 adults and using both 75 g oral glucose tolerance test (OGTT) and glycated haemoglobin (A1c) as diagnostic criteria, one in nine Chinese had diabetes, one in three had obesity (general or central) closely associated with hypertension and dyslipidemia and one in two had prediabetes. Amongst those with diabetes, only 30 % were diagnosed and amongst the diagnosed, only 30 % were treated and amongst the treated, only 40 % were controlled, defined as A1c less than 7 % [4].
According to the Diabetes Epidemiology Collaborative Analysis of Diagnostic Criteria in Asia (DECODA) data, 30–50 % of subjects in the WP Region had metabolic syndrome, characterised by central obesity and clustering of cardiometabolic risk factors, and a strong predictor for future development of diabetes [5]. In 2008, 60–70 % of people in Tonga, Nauru and Cook Islands were considered obese [6]. In 2004, the sex-standardised diabetes prevalence was reported to be 13.0 % in men and 14.4 % in women in Nauru. The age-specific prevalence rose from 4.5 % in the 15–24 age group to 42.7 % in the 55–64 age group [7]. Tables 5.2 and 5.3 summarise the prevalence and number of people with diabetes in the WP countries based on the IDF figures published in 2013 [8].
Table 5.2
Top ten countries in the Western Pacific Region in terms of number of subjects affected by diabetes [8]
Country/territory | Number of subjects (in 1000s), 2013 |
|---|---|
1. China | 98,407.379 |
2. Indonesia | 8554.165 |
3. Japan | 7203.776 |
4. Republic of Korea | 3323.903 |
5. Vietnam | 3299.113 |
6. Philippines | 3256.215 |
7. Thailand | 3150.670 |
8. Myanmar | 1988.846 |
9. Malaysia | 1913.236 |
10. Taiwan | 1721.062 |
Country/territory | Prevalence (%), 2013 |
|---|---|
1. Tokelau | 37.49 |
2. Federated States of Micronesia | 35.03 |
3. Marshall Islands | 34.89 |
4. Kiribati | 28.77 |
5. Cook Islands | 25.66 |
6. Vanuatu | 23.97 |
7. Nauru | 23.29 |
8. French Polynesia | 22.41 |
9. New Caledonia | 19.49 |
10. Guam | 19.48 |
Rapid quantitative and qualitative changes in nutritional intake, notably a shift from a traditional high-fibre, low-fat diet to an energy-dense diet with increased meat and fat consumption as well as reduced physical activity due to mechanisation and increased use of mobile vehicles, are important drivers for this global epidemic [9]. In the islands of Vanuatu, researchers reported close associations of economic development with increased consumption of animal proteins and simple carbohydrates, as well as increased tobacco and alcohol consumption in men in areas with high tourism [10]. In China, during the last two decades, changes in food technology with production of high-calorie foods together with reduced physical activity have contributed to the rising burden of obesity in both adults and children [11].
Unique Aspects of Pathophysiology: Beta-Cell Function Versus Visceral Obesity
Age, family history and obesity are the main risk factors for diabetes [4] although inadequate beta-cell response to overcome insulin resistance due to factors such as ageing, obesity and inflammation remains the primary culprit [12]. In an analysis of 74 study cohorts comprising 3813 individuals (19 African, 31 Caucasian and 24 East Asian cohorts), the authors examined the hyperbolic relationship between insulin sensitivity index (IS) and acute insulin response to glucose (AIRg) in healthy cohorts. Amongst these three subpopulations, Asians had a steep and non-linear relationship with small changes in one variable having large effect sizes on the other. Besides, Asians had the lowest body mass index (BMI) and AIRg and developed diabetes only with a small increase in BMI compared to the Caucasians and Africans [13].
Compared to their Europid counterparts, people of Asian ethnicity are more prone to develop diabetes for the same level of BMI or waist circumference due to their propensity to store excessive body or visceral fat [14]. Even amongst lean subjects and given the same amount of visceral fat, Asians were more insulin resistant than Europids due to increased concentration of free fatty acids and inflammatory markers [15]. Using insulin clamp studies, researchers from Thailand reported insulin deficiency as the predominant feature in lean subjects and insulin resistance and in the overweight and obese subjects [16].
In autopsy studies, Korean researchers have reported close correlations between BMI and relative percentage of beta cells in both diabetic (r = 0.55) and nondiabetic specimens (r = 0.35). However, diabetic subjects had a lower percentage of beta cells than nondiabetic subjects for the same BMI [17]. In healthy subjects, both Chinese and South Asian subjects with normal glucose tolerance exhibit higher glucose excursion during an OGTT with reduced rate of glucose disposal than their Caucasian counterparts [18]. This inherently low beta-cell function together with propensity to develop obesity which will impose metabolic stress has provided a highly plausible explanation for the high risk of diabetes in Asian populations undergoing rapid transition (Fig. 5.2).
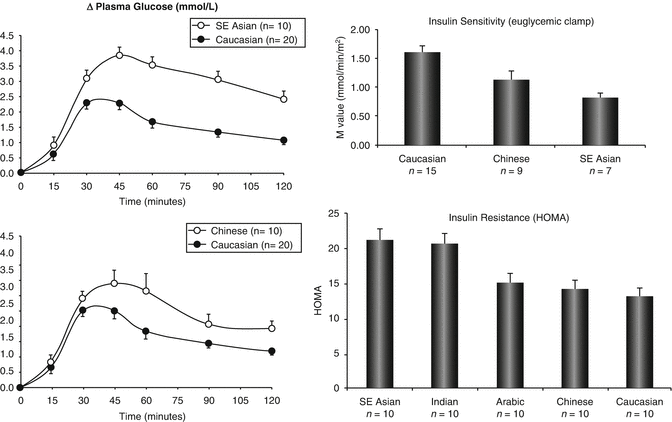

Fig. 5.2
Lean Chinese and Southeast Asians (open circle) with normal glucose tolerance exhibited higher glucose excursion during a 75 g oral glucose tolerance test and reduced insulin sensitivity during euglycaemic clamp studies compared to their Europid counterparts (black circle), supporting their biological vulnerability to develop diabetes under conditions of metabolic stress [18]
Genomic Landscape of Diabetes in the Region
Most of the genetic variants discovered in the genome-wide association studies (GWAS) in Caucasians have been replicated in Asian populations, albeit often with subtle differences in locations and frequency of genetic variants given the same locus [19] and larger effect size on beta-cell function in Asians for the same variant [20]. Besides, GWAS performed in Japanese, Chinese and Caucasian populations have revealed significant interethnic differences in genomic architecture as well as novel genetic variants in Asian populations which are implicated in protein synthesis and developmental and beta-cell biology [19, 21, 22]. Some of these novel variants were first discovered in patients with familial young-onset diabetes, suggesting that more variants may be discovered in these populations [23, 24]. These findings also support the notion that insufficient beta-cell response to rapid weight gain with progressive personal affluence through multiple generations [19] may contribute to this Asian epidemic with increasingly young age of onset. On the other hand, monogenic diabetes and latent autoimmune diabetes in adults [25] are not uncommon in young Asian populations which further increase the genotypic and phenotypic heterogeneity of diabetes in Asian populations [26] (Fig. 5.3).
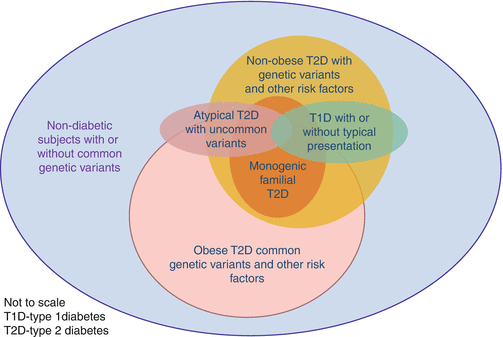

Fig. 5.3
A schematic diagram showing the phenotypic and genotypic heterogeneity of diabetes in Asian populations with complex interactions between genetic, autoimmune and external risk factors
In a recent epigenome-wide association study of South Asians and Caucasians, the methylation levels of several genetic loci associated with lipid metabolism and beta-cell biology were found to be associated with increased risk of diabetes in a dose-dependent manner [27]. These findings are in line with the preponderance of noncoding variants linked to diabetes in GWAS, suggesting that dysregulation of expression may be more important than protein changes as a genetic cause for the common form of diabetes [28]. In this light, maternal obesity and gestational diabetes may influence the in utero environment to cause dysregulation of expression of interlinking biological networks through DNA methylation and chromatin modification and contribute towards the high prevalence of childhood metabolic syndrome [29] and young-onset diabetes in Asian population [30, 31]. In this context, the ‘thrifty genotype or phenotype’ theory which states that a trait conducive to survival during energy-scare condition may become maladaptive in an energy-abundant environment may be particularly relevant to emerging economies like China and the WP Region [32]. In support of this notion, early-life exposure to the 1950–1960 famine in China was found to be associated with increased risk of diabetes and metabolic syndrome [33] especially in subjects with low birth weight and adult obesity [34, 35].
Nature Versus Nurture
The combination of low BMI and obesity has put Asians at high risk of diabetes although many of these risk factors are modifiable [36]. First and foremost, health illiteracy and social disparity [37] remain the most important socioeconomic determinant for diabetes and obesity [38, 39]. In these subjects, lack of awareness, poor education, psychosocial stress [40, 41] and long working hours [42] may amplify the adverse effects of behavioural risk factors such as poor sleep hygiene [43] and the use of tobacco [44], alcohol [45] and sugar-sweetened beverages [46] which can unmask diabetes especially in those with genetic predisposition [47].
Amongst these risk factors, low-grade inflammation [48] associated with endemic infections such as chronic hepatitis B viral infections affecting over 10 % of the Asian population [49, 50] as well as beta-cell dysfunction associated with the use of tobacco, with smoking rates as high as 50 % amongst Asian men from China, Japan, Korea and Indonesia [51], may be particularly relevant to the Asian environment. These external factors may interact with cultural factors such as high consumption of rice with high glycaemic index [52] to cause metabolic stress especially in those with compromised beta-cell function. Adding to this multicausality and complexity, environmental pollutants such as persistent organic pollutants (POPs) [53] and bisphenol A (BPA) [54] are endocrine or mitochondrial disruptors which tend to hit emerging economies and/or subjects with low socioeconomic status hardest. By inducing insulin resistance and/or beta-cell dysfunction, these chemicals may increase the risk of obesity and hyperglycaemia especially in subjects with genetic predisposition [54].
Morbidity and Mortality
According to the estimates by IDF in 2013, 36 % of global deaths in adults are due to diabetes affecting 5.1 million people. The most populous WP Region had the highest number of diabetes-related deaths affecting more men (1,080,000) than women (789,000). Of note, 44 % of deaths due to diabetes occurred in people under the age of 60 [1]. On average, diabetes reduces life expectancy by 6–12 years, especially in people diagnosed young, and increases the risk of vascular, cancer, nonvascular, non-cancer deaths by 1.3–3-fold. The nonvascular, non-cancer-related deaths are mainly due to renal failure, mental illnesses, hepatobiliary disease and sepsis with fasting plasma glucose having linear relationships with all clinical outcomes, even after adjustment for confounders such as age, sex, BMI and lifestyle factors [55].
In the WP Region, in contrast to Europids in whom cardiovascular disease is a leading cause of mortality and morbidity, diabetic kidney disease is a major complication in Asia [26], affecting 50–60 % of type 2 diabetic patients in different clinic settings [56]. In prospective studies, the annual incidence of diabetic kidney disease in Chinese population was 2–4 %, driven primarily by disease duration, smoking, metabolic syndrome, blood pressure and glycaemic control [57]. Other factors such as the use of over-the-counter medications including herbal medicine [58] and chronic hepatitis B infection [50] might also contribute to this high rate of diabetic kidney disease in the WP Region. While many patients in the WP Region die from end-stage renal disease due to lack of access to renal replacement therapy, this devastating condition can be prevented by intensive control of all risk factors using a protocol-driven and collaborative approach [59].
Countries in the WP Region differ considerably in the ecosystem of health-care provision and health literacy. In low-income areas such as some Pacific Islands where health illiteracy is common and access to care is poor, leg amputation continues to be a major cause of morbidity and mortality. In a recent survey of amputations involving 85 people with diabetes from Solomon Islands, Nauru and Vanuatu, delayed treatment (42 %), the use of traditional treatments (18 %) and insufficient knowledge about foot care (11 %) [60] are the main reasons for amputation.
On the other hand, several local, national and regional registries have provided insights regarding the interethnic differences in diabetic complications and the impact of ageing and care provision on the secular changes of these clinical outcomes. Apart from renal failure, stroke is a major disease burden in Asian diabetic populations [61]. Depending on the demographic pattern and stage of societal transition, cancer is now emerging as a leading cause of death especially in ageing societies and areas with high rate of endemic infections (e.g. hepatitis B for liver cancer) and high survival rates from cardiovascular-renal complications [62]. In the Australian National Diabetes Registry of nearly one million subjects, researchers have reported 1.3-fold increased standardised incidence risk of all-site cancer in both type 1 and type 2 diabetes except for prostate and melanoma [63]. In a national insurance database from Taiwan, diabetes-related death rates have declined over time (men, women: 3.92 %, 3.29 % in 2000; 3.64 %, 3.11 % in 2005; and 3.12 %, 2.71 % in 2009). In 2009, the estimated loss of life due to diabetes was 6.1 years in women and 5.3 years in men amongst people diagnosed at the age of 40. The four major causes of death were diabetes, malignancies, heart disease and cerebrovascular disease [64].
In the Hong Kong Diabetes Registry established as a quality improvement programme since 1995, researchers were able to track the secular changes in clinical outcomes in Chinese adults with diabetes, captured by a territory-wide clinical management system. In the early 1990s when universal health-care coverage was still being developed, stroke and end-stage renal failure were the leading causes of death. With ageing, societal affluence and access to interventions, coronary heart disease, cancer and heart failure became the leading causes of death. In 2005 and after a follow-up period of 5.5 years, amongst the 7534 type 2 diabetic patients enrolled in the registry since 1995, 763 died with the main causes of death being neoplasms (24.5 %) and cardiovascular (23.5 %), respiratory (15.3) and renal disease (15.3 %) [65].
Emerging Epidemic of Young-Onset Diabetes and Its Comorbidities
In the WP Region, 32,500 children under the age of 15 were estimated to have type 1 diabetes, with the largest number living in the Philippines (7900), followed by China (7700), while Australia has the highest estimated incidence rate of 22.3 cases per 100,000 children. In 2013, 5300 children were diagnosed to have type 1 diabetes in the WP Region [1]. Although type 1 diabetes is often considered a rare disease in non-Caucasian populations, the rising prevalence of young-onset type 2 diabetes is a major health-care challenge. Arbitrarily defined as age of diagnosis less than 40 years, these youths and young adults are high-risk populations for premature noncommunicable disease (NCD) including but not limited to cardiovascular-renal disease and cancer. This growing epidemic of young-onset diabetes is in part driven by the high prevalence of maternal obesity, gestational diabetes and childhood metabolic syndrome which sets up a vicious cycle of ‘diabetes begetting diabetes’ with enormous implications on the individual, family and society [66]. Between 2007 and 2014, the number of people with diabetes worldwide has increased by 74 % in the 20–39 years age group, from 38 million to 67 million. This compares to a 63 % increase in the 40–59 years age group and a 43 % increase in the 60–79 years age group with an especially sharp increase in the WP Region and Southeast Asia where China and India are located (Fig. 5.4).
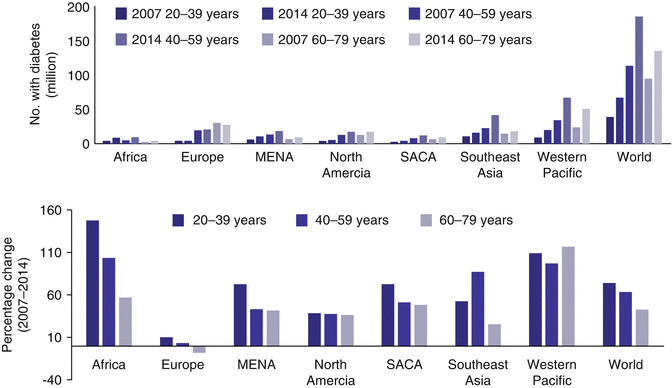

Fig. 5.4
Rates of increase and number of people with diabetes in different age groups showing the growing epidemic of young-onset diabetes especially in Asia (Adapted from IDF Atlas 2015 [1]) (MENA Middle East and North Africa, SACA South America and Central America)
In the same registry, the researchers reported the rarity of classical type 1 diabetes which accounted for 3 % in the entire cohort and less than 10 % amongst those diagnosed before the age of 40. Overall, one in five adults with type 2 diabetes had young-onset disease, with 50 % higher risk of cardiovascular-renal disease than their late-onset counterparts at any given age, mainly driven by long disease duration [67]. Amongst these young-onset type 2 diabetic patients, 40 % were lean and 60 % were obese or overweight. Compared to their counterparts with type 1 diabetes, these young-onset type 2 diabetic patients had considerably higher rates of cardiovascular-renal complications, often due to poor control of risk factors, frequent default and treatment non-adherence. By contrast, due to their propensity to develop an acute ketotic presentation, type 1 diabetic patients were less likely to default, and with access to education and insulin, these patients tended to have low incidence of complications despite long disease duration [68].
In a regional database established through the web-based Joint Asia Diabetes Evaluation (JADE) programme which enables care providers to establish registries using a common protocol in a real-world setting, high prevalence of young-onset diabetes (~20 %) and treatment gaps was confirmed in 41,029 adult patients from over ten countries in Asia. These real-world data highlight the heterogeneity in terms of attainment of treatment targets often due to differences in health-care coverage and financial support for essential laboratory investigations and medications as key components for diabetes care. Yet irrespective of these diversities, in all countries, there was a consistent trend that patients with young-onset diabetes were less likely to attain treatment targets for blood glucose and blood lipids and were less likely to be prescribed organ-protective drugs such as statins and renin-angiotensin system (RAS) inhibitor despite having clear indications such as cardiovascular-renal complications [30].
Diagnosis of Diabetes and Prediabetes in Asia
The criteria for diagnosing diabetes and prediabetes in Asia for the most part take references from international guidelines including those of the World Health Organization (WHO) and American Diabetes Association (ADA) [69, 70]. The measurement of fasting plasma glucose for diabetes screening is widely accepted and practised. On the other hand, there are differences in regional recommendations for oral glucose tolerance test (OGTT) and glycated haemoglobin (A1c) in the routine diagnostic process. Approaches to screening are also closely related to health-care access and resources available. For instance, the Chinese Diabetes Society guideline for the prevention and control of type 2 diabetes last updated in 2010 has not yet endorsed the use of HbA1c as an alternative to glucose-based tests for diagnosing diabetes [71]. Similarly, the UNITE FOR Diabetes Philippines in its 2014 practice guideline also recommended against using A1c. They also proposed the use of OGTT for diabetes screening only in individuals with known impaired fasting glucose or metabolic syndrome [72]. In the latest report published in 2010, the Japan Diabetes Society adopted the use of A1c to complement glucose-based tests in diabetes screening but stipulated that an individual meeting the A1c threshold of 6.5 % must concurrently fulfil one other criteria of either fasting plasma glucose ≥7.0 mmol/L or 2-h post-OGTT plasma glucose ≥11.1 mmol/L to establish the diagnosis [73]. This differs from international guidelines which assign similar weights to glucose-based studies and A1c in the diagnostic schema and allows the diagnosis to be made on the basis of A1c measurement alone. The Korean Diabetes Association fully applied the ADA criteria for diagnosing diabetes since 2011 [74].
Glycated haemoglobin has a number of advantages over plasma glucose in that it summarises long-term glucose exposure, has less intra-individual variability and does not require testing during a fasted state [75]. The recommendation to include A1c in diagnostic criteria of diabetes by the international expert committee was based on epidemiological studies which demonstrated a tight association between A1c and diabetic retinopathy in mixed populations [75–77]. Progress in analytic procedures and global movement to certify A1c assay to the National Glycohemoglobin Standardization Program facilitated its wider use as a diagnostic tool as well as in disease monitoring [75, 78]. In developing countries such as China and the Philippines, however, the measurement of A1c has not yet achieved standardisation across the nation, and this remains the key limitation to its application [72].
Notwithstanding logistics of costs and instrumentation, inclusion of A1c in diagnostic criteria will uncover more patients with diabetes than using glucose-based criteria alone. In large population surveys in China and Japan, over half of the participants with newly diagnosed diabetes and prediabetes were detected with A1c. This may be due to the high prevalence of high post-glucose challenge blood glucose levels which may be detected by an integrated value like A1c but missed by using fasting plasma glucose only [4, 79].
Increasingly, it is recognised that ethnicity may modify the relationship between measured A1c and blood glucose levels. Several studies have shown that for similar distribution of blood glucose, A1c levels were consistently higher in Asians compared to Caucasians and that amongst Asians, higher in Indians and Malays relative to Chinese [80, 81]. A few factors may contribute to such racial disparities such as differences in haemoglobin glycation rate and in population prevalence of haemoglobinopathies which interfere with A1c measurement.
Against this background, concerns are raised regarding the optimal A1c threshold for detecting diabetes in Asian groups and whether ethnic-specific standards are required. Studies carried out in Asians have suggested that lower cut-offs of A1c correlated more closely with diabetes as defined using the gold standard of OGTT [82–84]. In a recent population-based study comprising three ethnic groups of Malay, Chinese and Indian which examined the cross-sectional relationship between A1c and moderate retinopathy, it was found that although A1c differed slightly in its sensitivity and specificity in predicting retinopathy amongst different ethnic groups, eye complication was generally rare when A1c fell below 6.5 % [85]. Results from this Asian study concurred with those of a large multinational database which confirmed that an A1c threshold of 6.5 % has similar discriminatory power for the presence or absence of diabetic retinopathy in Caucasians and Asians [77, 85].
Oral glucose tolerance test is the gold standard test for the diagnosis of diabetes and is necessary in making a diagnosis of impaired glucose tolerance (IGT). The requirement of a second blood sample and being more time consuming has greatly limited its practice in many Asian countries. However, the importance of the 2-h plasma glucose has been highlighted in studies conducted in Asia which showed that fasting plasma glucose alone could only identify half of the people with diabetes and that individuals with predominantly post-load hyperglycaemia are phenotypically different from those with fasting hyperglycaemia [86]. As discussed, Asian diabetic patients have lower BMI and less beta-cell reserve than their Caucasian counterparts and thus are more likely to fail glucose stress test. On this premise, a lower threshold to perform OGTT in Asians should be considered. In Chinese, a paired value of A1c ≥6.1 % and fasting plasma glucose ≥6.1 mmol/L had 13–17 times increased likelihood to occur in diabetic subjects compared to nondiabetic subjects [87, 88]. In resource-limited setting, OGTT will have the greatest yield in detecting diabetes when paired A1c and fasting plasma glucose are above these cut-off values.
Translating Primary Prevention of Type 2 Diabetes
The chronic, silent and nonurgent nature of diabetes often leads to late diagnosis and delayed treatment. Once diagnosed, people with diabetes have pluralistic needs including information, social and psychological support, education, medical and surgical treatment, to name but a few. Given these multiple demands, large population size, lack of capacity and resource restraints, prevention and control of diabetes is often challenged with care fragmentation, clinical inertia and treatment non-adherence with insufficient community support [3, 89, 90]. In both randomised disease management programmes and observational cohorts, attaining multiple treatment targets and the use of organ-protective drugs such as statins and RAS inhibitors have been shown to reduce cardiovascular-renal complications and related deaths in the WP Region [91, 92]. Yet large-scale surveys have demonstrated only 30 % of patients with diabetes ever had assessments for risk factors and complications. Amongst those who had assessments, only 30–40 % attained one of the three ABC targets (A1c <7 %, BP <130/80 mmHg, LDL-C <2.6 mmol/L) and 5–10 % attained all three targets [93] with particularly low rates amongst people with young-onset diabetes [30, 94].
Diabetes is a lifelong disease which requires acquisition of knowledge, change of attitudes and formation of new habits in order to reduce disease burden. It is also a biological problem that requires medications, technologies and monitoring which have resource implications. In a survey from Guam involving 125 patients, 40 % were not aware of the type of diabetes they had, 20 % had not received education on self-management and 30–60 % had not received advice on tobacco cessation, immunisation services, nutritional counselling and/or regular eye and foot examinations. Over 50 % of patients expressed their desire to have preventive and education services with enhanced access to specialists and specialised care as well as financial support to cover the costs of chronic care and medications [95].
There is now consensus that a chronic care model involving community mobilisation and engagement supported by an integrated health-care system with country/area appropriate financing and health-care organisation is needed to promote interactions between an informed person with diabetes and a proactive health-care team to achieve positive clinical outcomes [96]. In this information and technology era, there is a growing advocacy to use improvement science, often entailing the use of real-world data, to inform and change clinical practice [97]. In the case of diabetes, changing the workflow and using protocols to collect data systematically for establishing registry can generate personalised report to promote shared decision-making. On a broader perspective, data transparency and comparison of performance indexes may also motivate practice and policy changes through ongoing evaluation and sharing of best care models [98–100].
Western Pacific Diabetes Declaration (WPDD) Plan for Action (2006–2010)
In 2000, at the 51st session of the WHO Regional Committee for the WP, the Committee endorsed the establishment of the WPDD as a regional strategic alliance between the WHO Regional Office for the WP, the Secretariat of the Pacific Community and the IDF-Western Pacific Region as a regional voice to advocate for prevention and control of diabetes. In the WPDD Plan of Action (2005–2010), experts from the region summarised the multidimensional nature of diabetes (Fig. 5.5) and the need to use a multipronged strategy including policies and legislations (Fig. 5.6) to create a health-enabling environment and reform the health-care system to make prevention and control of diabetes accessible, affordable and sustainable [101].
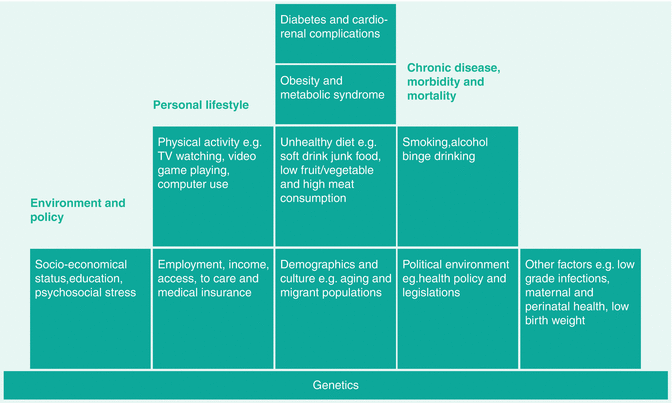
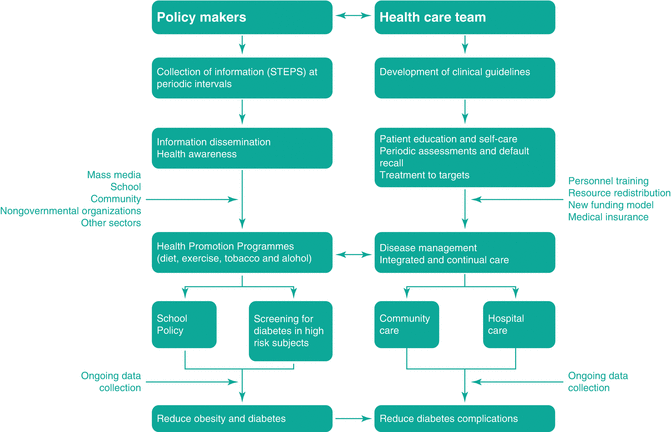

Fig. 5.5
A schematic diagram summarising the interactions amongst predisposing, precipitating and perpetuating factors in the epidemic of diabetes and its comorbidities in the Western Pacific Diabetes Declaration – Plan for Action (2005–2010) [154]

Fig. 5.6
A multipronged strategy using policies, community mobilisation, intersectoral partnerships and clinical leaderships to detect, prevent and control diabetes in the Western Pacific Diabetes Declaration – Plan for Action (2005–2010) [154]
Some Examples of Diabetes Prevention and Control Strategies from China and WP
National Plan for Diabetes and NCD Prevention
In the China National Plan for NCD Prevention and Treatment (2012–2015), the government proposed to use public measures including environmental protection, food safety, clean drinking water, tobacco control and occupational safety to create a health-enabling environment through broad social participation and multisector collaborations. Other proposed practices and policies included universal vaccination, maternal and child health programmes and primary care. Since then, the Chinese government has introduced different health insurance packages at the rural and urban areas and built more than 30,000 community-based clinics to provide basic preventive care programme including regular risk factor surveillance and provision of essential medications for control of blood pressure and blood glucose [102]. Despite these ongoing efforts, given the vastness of the country and size of the population, there remain challenges in communications amongst relevant stakeholders, patient flow between hospitals and primary care clinics, capacity building and public education [103].
Tobacco Control
Several meta-analyses have indicated a dose-dependent risk association of smoking with diabetes [104] as well as that with cardiovascular disease and total mortality in people with diabetes [105]. Although it remains to be confirmed whether smoking cessation will reduce the risk of diabetes, it is likely that tobacco control would reduce the amplifying effects of tobacco on morbidities associated with diabetes, notably, cancer and cardiovascular-renal disease. To this end, there have been successful stories in tobacco control through strong political will and tough measures in the WP Region, such as New Zealand and Hong Kong [106].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree