Chapter 71 Diabetes and Insulin Resistance
INTRODUCTION
Insulin resistance and type 2 diabetes mellitus are increasingly recognized as common among individuals with HIV infection who are receiving antiretroviral therapy.1–3 One important consequence of diabetes and insulin resistance is the associated increased risk of cardiovascular disease in HIV-infected patients.4 In a large prospective cohort study, Friis-Moller and colleagues4 demonstrated increased rates of acute myocardial infarction with increased duration of exposure to HAART, such that each year of HAART use was associated with a 1.26 (95% CI 1.12–1.41, P < 0.001) relative increase in risk for acute myocardial infarction (AMI). In addition to the risk of HAART, traditional independent risk factors for cardiovascular disease were identified and diabetes was associated with a 2.38-fold increased relative AMI risk (95% CI 1.38–4.10, P =0.002). These findings suggest that insulin resistance and diabetes may contribute to cardiovascular disease among HIV-infected patients. This chapter summarizes new data on the prevalence of diabetes and insulin resistance in HIV-infected patients, summarizes our understanding to date of the mechanisms of insulin resistance and diabetes in the HIV-infected population and highlights a rational approach to the management of diabetes and insulin resistance in HIV-infected patients.
DEFINING DIABETES, IMPAIRED GLUCOSE TOLERANCE, AND INSULIN RESISTANCE IN THE HIV-INFECTED POPULATION
Diabetes is defined by the WHO as a fasting plasma glucose equal to 126 mg/dL or more, or a 2-h glucose 200 mg/dL or more during a 75 g oral glucose tolerance test (OGTT).5 Impaired glucose tolerance (IGT) is defined as a 2-h plasma glucose 140 mg/dL or above during a 75 g OGTT,5 and impaired fasting glucose (IFG) is defined more recently as a fasting plasma glucose 100 mg/dL or more.6 Both diabetes and IGT are associated with increased cardiovascular risk.7–10 In addition, patients with IGT have a substantial risk of going on to develop diabetes. In a prospective study of over 3000 individuals, the Diabetes Prevention Program found an estimated cumulative incidence of diabetes of 29% after 3 years of follow-up for subjects with IGT who did not receive any intervention.11
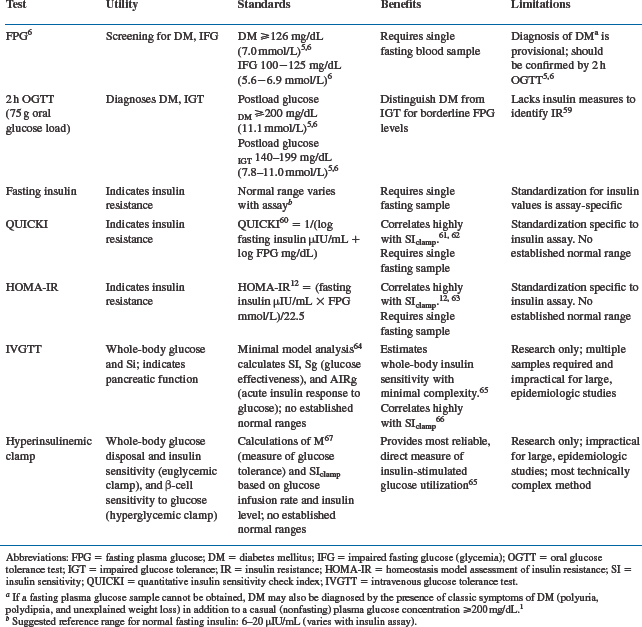
Insulin resistance is more difficult to define clinically, but is usually marked by hyperinsulinemia, and decreased insulin action (e.g., decreased insulin-mediated glucose transport into muscle and impaired insulin action to inhibit lipolysis). Numerous tests exist to determine abnormal glucose homeostasis, as listed in Table 71-1. A simple fasting blood glucose or 75 g OGTT are most useful clinically to diagnose diabetes or IGT. A fasting insulin level or index of fasting glucose and insulin (e.g., HOMA and QUICKI, see Table 71-1.) is relatively easy to obtain and can be useful for the evaluation of insulin resistance.12 More sophisticated techniques such as the euglycemic hyperinsulinemic clamp are not practical clinically, but remain important research tools to quantify insulin resistance. While useful in the care and monitoring of patients with known diabetes, the hemoglobin A1c, an integrated measure of the average glucose over the prior few months, is not a useful measure in patients with significant insulin resistance but normal fasting glucose levels, which is common in HIV infection.
PREVALENCE OF DIABETES AND ABNORMAL GLUCOSE HOMEOSTASIS INHIV-INFECTED PATIENTS
Early reports of hyperinsulinemia among HIV-infected men and women indicate that insulin resistance was prevalent even before the widespread introduction of HAART. For example, compared to weight-matched healthy controls, HIV-infected men and women with a history of wasting or prior weight loss demonstrated increased fasting insulin levels and markers of insulin resistance.13,14 In both studies, there was no association between insulin levels and the use of protease inhibitors (PIs), the component of HAART most often associated with insulin resistance. Furthermore, more than 40% of the men14 and 75% of the women13 were not on HAART regimens in these studies. Increased relative truncal adiposity was demonstrated in relationship to hyperinsulinemia even among a population of nonobese HIV-infected patients with a history of weight loss.13,14 Studies have also demonstrated insulin resistance among HIV-infected patients using HAART regimens containing a PI.15,16
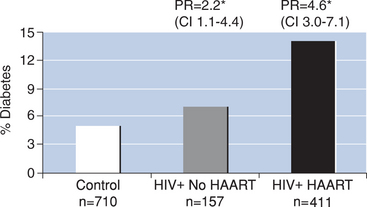
More recently, Brown et al2 investigated the prevalence and incidence of diabetes mellitus in a large cohort of HIV-infected men in the Multicenter AIDS Cohort Study. In this study of 710 HIV-seronegative men and 568 HIV-infected men, the prevalence of diabetes was significantly greater among HIV-infected men based on a fasting glucose above 126 mg/dL or self-reported diabetes mellitus (overall prevalence of diabetes: 12% HIV+ vs 5% HIV−). When subjects were further categorized according to current use of HAART, HIV-infected men not receiving HAART demonstrated a prevalence ratio of 2.21 (95% CI 1.12–4.38) compared to controls. In contrast, men receiving HAART demonstrated a prevalence ratio of 4.64 (95% CI 3.03–7.10) compared to controls, after adjusting for age and body mass index. Similarly, the incidence of diabetes between April 1999 and March 2003 was significantly increased among the HIV-infected men receiving HAART compared to HIV-seronegative controls (incidence rate: 4.7 per 100 person-years and the adjusted rate ratio was 4.11 (95% CI 1.85–9.16)). In a subanalysis to identify potential risk factors associated with diabetes mellitus and IGT, ritonavir was the only PI associated with increased risk (rate ratio 1.70 (95% CI 1.08–2.68)) of diabetes or IFG (i.e., fasting blood glucose between 100 and 125 mg/dL) when considered together. However, 94% of the participants using ritonavir were also receiving another PI in combination with ritonavir, which was used for its ‘boosting effects’ to improve the pharmacokinetic profile of other PIs, making it more difficult to interpret these results. Brown et al also demonstrated that men with a lower CD4 nadir had a greater risk of incident glucose abnormalities, suggesting that severity of HIV disease may be related to an increased risk of diabetes or insulin resistance. In a 4-year follow-up period, this study identified newly diagnosed diabetes mellitus in 10% of HIV-infected men on HAART. A fourfold increased incidence of diabetes compared to HIV-seronegative men was observed after adjusting for age and body mass index.
The risk and prevalence of diabetes and insulin resistance among women with HIV infection is less clear. Justman and colleagues3 reported a threefold increase of incident diabetes mellitus among HIV-infected women receiving a PI compared to women receiving nonPI-based antiretroviral therapy in a cohort of 1785 women followed in the Women’s Interagency HIV Study (WIHS) between 1994 and 1998. In addition to PI use, increasing age and body mass index were identified as significant independent risk factors for self-reported diabetes mellitus.3 More recently, Howard et al17 reported OGTT results from a large cohort of HIV-infected women (n = 133) and HIV-uninfected, age-matched controls (n = 88) without a history of diabetes. Here, 13.5% of HIV-infected women demonstrated IGT (i.e., 2-h postchallenge glucose level ≥140 mg/dL and <200 mg/dL) and 4.5% demonstrated previously unrecognized diabetes. Rates between the HIV-infected and HIV-uninfected groups did not differ significantly, but women in the control group had higher body mass index and waist circumference, and therefore may have had greater risk for abnormal glucose metabolism.
Danoff et al18 published a subsequent study of women enrolled in the WIHS cohort, evaluating prospective oral glucose tolerance testing in 258 women. The study tested 88 HIV-seronegative, 74 HIV-infected women not receiving HAART, and 96 women with HIV infection receiving HAART. Fourteen percent of the HIV-infected participants had ‘prediabetes’ defined as a fasting blood sugar above 100 mg/dL or a 2-h postchallenge glucose level between 140 mg/dL and 200 mg/dL. The prevalence of diabetes was not increased in the HIV-infected women receiving HAART (4%) or the HIV-infected women not receiving HAART (8%) compared to the control women (10%), but this study also reported data from relatively overweight control subjects enrolled in the WIHS study. Indeed, in the study of Danoff et al, body mass index emerged as the single most important predictor of diabetes and prediabetes.18 In summary, abnormalities in glucose metabolism may be seen in up to 14% of HIV-infected women, but the limited data currently available do not yet support an increased prevalence of diabetes mellitus in women living with HIV compared to a well-matched HIV-uninfected control group.
Although fewer data are available in children, preliminary studies indicate that insulin resistance may be increased, particularly among HIV-infected children on HAART. For example, in a small cross-sectional study of HIV-infected children on a PI-containing regimen (n = 33) compared to PI-naive children (n = 15), Bitnun and colleagues19 identified lower insulin sensitivity in children after adjusting for potential confounders. In a larger, multicenter, 2-year prospective study of 130 HIV-infected children designed to assess lipodystrophy and associated metabolic complications, Beregszaszi et al found 13.2% of children to have hyperinsulinemia (defined as an insulin level greater than the 95th percentile from a normoglycemic healthy population matched for pubertal status).20 After 2 years of follow-up, this percentage doubled to 25.6% (P = 0.01). Although children in the study were not found to have diabetes mellitus by fasting hyperglycemia, fasting hyperinsulinemia was common and could represent an early stage in the ultimate development of diabetes among HIV-infected children.
RISK FACTORS FOR INSULIN RESISTANCE AND DIABETES IN HIV
The use of PIs was one of the first factors identified with increased risk of insulin resistance and diabetes among HIV-infected patients. Recognition of lipodystrophy and metabolic complications associated with antiretroviral therapy occurred with the widespread introduction of PIs and HAART. However, increasing evidence suggests that, while antiretroviral medications can have direct effects on insulin sensitivity and glucose metabolism, e.g., via inhibition of glucose uptake into muscle by individual PIs,21 indirect effects of antiretroviral medications on body fat distribution and lipids can contribute to impaired insulin sensitivity in HIV-infected patients. HIV infection itself may also contribute to chronic inflammation, and insulin resistance in this regard. Finally, changes in body composition, including truncal adiposity, may be associated with inflammatory cytokines and adipocytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and adiponectin, which may further contribute to insulin resistance in HIV-infected patients.22
In an early cross-sectional study designed to compare HIV-infected patients with evidence of fat redistribution to age and BMI-matched subjects from the Framingham Offspring Cohort, 71 HIV-infected men and women complaining of changes in body fat distribution and 213 healthy age, sex, and weight-matched control subjects were studied.1 HIV-infected patients with abnormal fat distribution had significantly increased rates of previously unrecognized diabetes (7% vs 0.5% in the control subjects) and increased rates of IGT (35.2% vs 5.2% in control subjects). In a subanalysis of 30 HIV-infected subjects without changes in body fat, there was no increase in diabetes or IGT compared to 90 age, sex, and weight-matched control subjects. These data suggest that fat distribution per se is an important factor in the development of insulin resistance among HIV-infected patients, who demonstrated increased central adiposity and waist-to-hip ratios compared to control subjects.
Lipoatrophy of the peripheral subcutaneous fat, as well as increased visceral fat, may contribute to insulin resistance in HIV. Mynarcik et al23 identified an association between insulin sensitivity and peripheral limb fat in a study of HIV-infected subjects with and without lipodystrophy in comparison to healthy volunteers. Reduced limb fat was associated with increased insulin resistance, similar to the observation in HIV-uninfected patients with congenital lipodystrophy. Lipoatrophy is increasingly recognized as a direct effect of antiretroviral therapy, including nucleoside reverse transcriptase inhibitors (NRTIs).24 Indeed, cumulative exposure to NRTIs and not PIs was identified as the strongest drug class association with markers of insulin resistance in the Multicenter AIDS Cohort Study of 755 HIV-infected men.25 The putative mechanism for this observation is through lipoatrophy and mitochondrial toxicity to adipose tissue. In addition, mitochondrial toxicity to muscle may also contribute to insulin resistance, via increased intramyocellular lipid accumulation.
Several studies have also identified a strong positive relationship between visceral fat and insulin resistance in HIV disease.13,19,26–28 For example, using positron emission tomography (PET) with administration of 2-Deoxy-2 [18F] Fluoro-D-glucose, insulin stimulated glucose uptake in subcutaneous and visceral fat and skeletal muscle were assessed in patients with HIV-associated lipoatrophy and healthy controls.28 Under insulin-stimulated conditions, visceral adipose depot size was correlated most significantly with whole-body glucose uptake (see Fig. 71-1), as well as regional muscle and fat glucose uptake. Therefore, as demonstrated in the general population with obesity and the metabolic syndrome, increased visceral adiposity may be an important determinant of insulin sensitivity in the HIV-infected population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree