(1)
Mayo Clinic, Jacksonville, Florida, USA
1.1 Introduction
By 2013, the world’s population reached 7.2 billion with 138.8 million annual births and 54.9 million annual deaths [145]. The global population is projected to continue to increase, reaching 8.3 billion people by 2013, with 13% of these people falling into the fastest-growing age group – those over the age of 65 [119]. The world’s changing demographic profile results from a remarkable transition in the causes of death. No longer are people dying primarily from communicable diseases. Rather, most deaths today are the result of noncommunicable diseases, a shift that has dominated the statistical patterns of industrialized countries for decades and is now also the trend in low- and middle-income countries.
Between 1970 and 2010, global life expectancy increased from 56.4 to 67.5 years for males and from 61.2 to 73.3 years for females [162]. During this same period, age-specific death rates were declining. Longer life expectancy prolongs exposure to various environmental and age-related risk factors and increases the incidence of deaths from noncommunicable diseases. In 2013, more than two-thirds of global deaths were due to noncommunicable diseases, with 17.3 million deaths due to cardiovascular and circulatory diseases, 8 million deaths due to cancer, and anywhere from 1.5 to 5.1 million deaths due to diabetes mellitus, making diabetes the eighth leading cause of death worldwide [30, 154]. Among older adults, diabetes is most heavily concentrated in Oceania, Latin America, North Africa, and the Middle East where a high body mass index is an important risk factor. High blood pressure is an important risk factor in Central and Eastern Europe, whereas high body mass index is important in Latin America, Oceania, North Africa, and the Middle East [98]. Of the 671 million obese individuals worldwide, 62% live in developing countries [122, 123]. The incidence rates of diabetes are likely to continue rising in association with the rapidly increasing obesity epidemic.
Diabetes mellitus (DM) represents a leading cause of morbidity and mortality throughout the world. Approximately 347 million persons worldwide (approximately 8.3% of the adult population) have diabetes mellitus [30, 154], and this has been projected to grow to 592 million by 2035 (Fig. 1.1) [12], numbers that have been revised upward several times in recent years. Diabetes ranks among the top ten causes of disability, primarily due to loss of ambulation from lower limb amputations and blindness due to diabetic retinopathy (DR). Because of its effects on pregnancy, DM adversely affects more than 21 million live births annually.
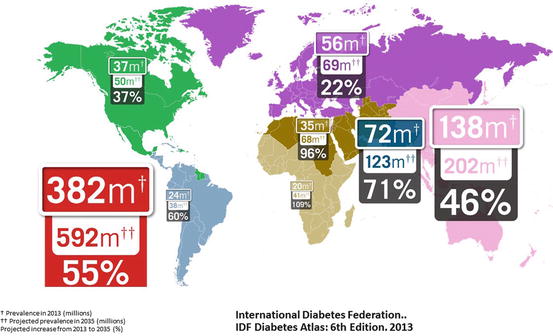

Fig. 1.1
This drawing shows projected increases in the prevalence of diabetes mellitus by continent from 2013 to 2035. The total number of diabetic patients throughout the world is projected to increase by 55% to 592 million. Increases are particularly significant in developing areas of Africa, the Middle East, and India
Diabetes disproportionately affects poor societies, as low- and middle-income countries account for 80% of diabetes-related mortality [72]. Diabetes-attributed mortality outstrips the combined annual deaths from malaria (600,000), tuberculosis (1.5 million), and AIDS (1.6 million) [169–171]. When combined with cardiovascular disease, cancer, and chronic respiratory diseases, diabetes accounts for an annual global death toll of 35 million – over 60% of the world’s mortality.
Worldwide concern over the increasing importance of DM even led to the passage of a 2006 United Nations resolution designating November 14 as “World Diabetes Day.” It was not the mortality rate, however, but rather the macroeconomic impact that prompted global action on diabetes and other noncommunicable diseases (NCDs). It has been estimated that for every 10% rise in the incidence of NCDs, a country loses 0.5% of its gross domestic product [152]. Noncommunicable diseases ranked among the top global risks to business due to the trillions of dollars incurred annually through lost productivity [168]. Diabetes incurs an annual healthcare cost of US$548 billion for 20–79-year-olds [30]. In low- and middle-income countries, blindness due to diabetes often disables breadwinners, burdens caregivers, and perpetuates a cycle of poverty.
Type 1 diabetes mellitus (T1DM) results from unopposed autoimmune destruction of the islet β-cells of the pancreas, which leads to insufficient production of insulin. Only 5–10% of worldwide cases of diabetes are classified as type 1 with the balance being type 2. Genetic factors appear to play important roles in the development of T1DM [112, 129, 153] but efforts to identify the responsible genes and their variants have met with limited success. More than half of the significant association signals for T1DM have been identified within chromosome 6p21 [15, 125, 129, 147], which maps to the human leukocyte antigen (HLA) region. This constitutes the most important region in the vertebrate genome regarding infection and autoimmunity and is crucial in regulating adaptive and innate immunity. A recent study of 4075 patients with T1DM identified 452 associated genes. Seven genes including four nonhuman leukocyte antigen (HLA) genes (RASIP1, STRN4, BCAR1, and MYL2) were replicated in at least one independent population and were differentially expressed in peripheral blood mononuclear cells or monocytes [136]. This emphasizes the fact that the genetic susceptibility pattern for T1DM appears complex [73] and may involve both susceptible and protective haplotypes. Despite our improved understanding of genetic associations, the triggering mechanism for the development of T1DM remains unknown, and in genetically susceptible individuals, it may result from exposure to as yet unidentified environmental factors.
The relative contributions of these haplotypes and their interactions with environmental factors and other genetic loci might partially explain the ethnic and racial variations in the frequency of T1DM [71]. Type 1 diabetes predominantly affects individuals of European ancestry, with the highest rates in Finland and Sardinia [11]. Asian and sub-Saharan African countries generally report low frequencies of T1DM, but Kuwait and China recently have reported higher rates [113, 180]. The SEARCH Study for Diabetes in Youth (2009) determined that T1DM remains a Caucasian-dominated disease with prevalence rates among 0–19-year-olds of 2.00 per thousand in non-Hispanic white patients, 1.31 for African-Americans, 0.99 for Hispanics, 0.94 for Navajos, and 0.52 for Asians and Pacific Islanders [11, 103].
Remarkable economic growth in Asia during the past 30 years has greatly improved the region’s standard of living and extended life expectancy. Growth is transforming the region from a predominantly low-technology, agrarian society toward an industrialized society with greater urbanization. This has increased food availability and caloric consumption and created a more sedentary lifestyle with a declining rate of communicable diseases. Obesity rates have increased as have the prevalences of T2DM, further straining the already challenged healthcare systems in the developing countries of the region.
Type 2 diabetes is characterized by peripheral insulin resistance, impaired regulation of hepatic glucose production, and declining ß-cell function that eventually leads to ß-cell failure and dependence on exogenous insulin [154]. With rapid growth in the prevalence of T2DM in the last two decades, there has been a surge in the reports of T2DM-related diabetic retinopathy (DR), especially from Asia. In 2030, the largest numbers of patients with diabetes will be in India and China. About 150 million people in China currently show early symptoms of diabetes [27]. By the year 2033, 80 million people in India will suffer from T2D [154] and 1 million people with diabetes will die every year [37, 56].
1.2 Incidence of Diabetes
Substantial data showing the prevalence rates of DM among specific ethnic and racial populations and within several countries has been published. There appears to be uniform agreement that the prevalence rates among most of these groups will significantly increase in the foreseeable future. Some authors have characterized the rate of increase in the global prevalence of T2DM, particularly in some areas of the globe, as alarming [157, 164]. It is estimated that the prevalence of diabetes among people over the age of 16 years will rise by 28.3% between 2010 and 2030, with 54.5% of this increase being attributed to increased obesity [2]. The incidence of T1DM is also rising but not to the same degree [11].
In 2008, there were an estimated 2.4 million Canadians (out of a population of approximately 30 million) with DM, and this is expected to increase to 3.7 million by 2018 [66]. Five and one-half percent of the adult population of France suffers from T2DM. Among affected patients, the average age is 65.9 years, 55% are men, the prevalence of obesity is 43%, and 18% take insulin [46]. The number of people with DM in the Middle East is expected to triple from the year 2000 to 2030 to approximately 60 million [148].
The prevalence of T2DM in South African adults rose from 5.5% in 2000 to 9.0% in 2009. Two million South Africans currently live with T2DM with 115,000 new cases estimated to occur each year [12]. Eye screening programs have been ineffective because an estimated 55% of adults with diabetes remain undiagnosed [65].
According to the International Diabetes Federation, Brazil’s population of patients with DM is the fourth largest in the world (11.9 million in 2013) with an estimated prevalence of 10.3% [8].
The annual age-adjusted prevalence and incidence rates of diabetes in the United States did not change significantly during the 1980s, but each rate increased yearly from 1990 through 2008 before leveling off until 2012. The prevalence per 100 population was 3.5 in 1990, 7.9 in 2008, and 8.3 in 2012 (Fig. 1.2). The incidence per 1000 persons was 3.2 in 1990, 8.8 in 2008, and 7.1 in 2012. Trends in many subpopulations were similar to these overall trends, but the incidence rates among non-Hispanic blacks (P = .03) and Hispanic adults (P = .01) continued to increase at rates significantly greater than those for non-Hispanic white adults. The prevalence rate increased faster for adults with a high school education or less, than for those with more than a high school education [57]. Based upon population and obesity trends, it was recently predicted that for a child born in the United States in 2000, the lifetime probability of being diagnosed with diabetes is 33% for males and 39% for females [117].
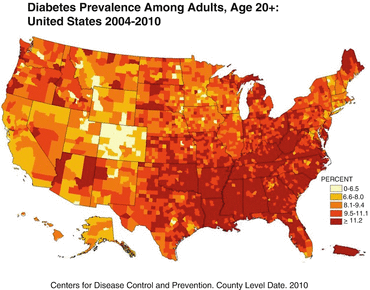

Fig. 1.2
This figure shows the county-by-county 2010 prevalence of diabetes mellitus in the United States. Note the very high prevalence rates in the southeastern United States, Puerto Rico, and scattered counties throughout western states in which there are high populations of Native Americans
For predisposed individuals who do not yet meet criteria for the diagnosis of DM, early changes in lifestyle can prevent the onset of T2DM. The Diabetes Prevention Program was a multicenter, randomized, controlled clinical trial that enrolled overweight individuals with elevated blood glucose levels but without definitive criteria for the diagnosis of diabetes. The consistent adoption of lifestyle changes including a low-fat diet, weight loss, and increased physical activity reduced the development of T2DM by 58% compared with placebo. Metformin (850 mg twice daily) lowered the incidence of diabetes by 31% compared with placebo. The study also demonstrated that approximately 8% of the patients who were prediabetic by commonly used clinical criteria already had diabetic retinopathy [7]. This suggests that the current criteria necessary for the diagnosis of DM exclude some patients who already show evidence of diabetes-related damage. Therefore, an argument can be made for changing the criteria required to diagnose T2DM.
Good control of blood glucose concentrations is the best way to prevent the development and progression of diabetes-related complications, but many patients with DM fail to achieve or maintain adequate metabolic control. Unfortunately, patients who manage to keep their HbA1c low have an increased risk of symptomatic hypoglycemia. The incidence of severe hypoglycemia in the Diabetes Control and Complications Trial (DCCT) was three times higher in the intensive treatment group compared with the conventional treatment group. Therefore, it appears unrealistic for patients with T1DM to pursue perfect glucose control because of the elevated risk of hypoglycemic episodes. Intensive glycemic control in the DCCT was also associated with a weight gain of 4.6 kg more than those in the conventional treatment group [51, 52]. It is difficult for patients with T2DM to achieve optimal metabolic control as patients in the intensive treatment group in the UKPDS were also plagued by increased hypoglycemic episodes and weight gain [51]. Metabolic control deteriorated over time in the UKPDS, possibly because of progressive loss of islet beta cell function [156]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study was stopped because of increased all-cause mortality in people whose glucose was extremely tightly controlled with insulin and multiple oral agents [1].
Obesity is a major risk factor for the development of T2DM [44, 55, 68, 97, 160], and increased prevalences of both diabetes and obesity are closely correlated [110]. The increases in obesity prevalence rates may have recently plateaued [50, 128] because of declines in overall food purchases and caloric intake, which could mean that the growth rate of T2DM may stabilize [53, 122]. Bariatric (weight loss) surgery is an effective and increasingly commonly used treatment for obese patients with T2DM [179].
Physical inactivity as part of a sedentary lifestyle is considered to be an important modifiable risk factor for T2DM and cardiovascular disease. Increased physical activity may slow the onset and progression of insulin resistance and improve glycemic control, blood pressure, and the lipid profile [69, 76].
1.3 Incidence of Diabetic Retinopathy
Visual impairment is a major public health problem that significantly diminishes the quality of life of affected individuals. Visually impaired patients report having difficulty reading, driving a car, and preparing meals [58, 105, 106, 161]. Vision impairment leads to higher incidences of social isolation, poor overall health, and falls. Not only does vision impairment impose a major burden on the affected individuals but also on their families, caregivers, and society.
Diabetes is the leading cause of vision loss in patients between the ages of 20 and 74 years in industrialized nations [33, 140]. Diabetes affects all parts of the eye, ocular adnexae, neurosensory pathway, and ocular motility system, but most DM-related vision loss stems from retinopathy due to microvascular complications. Early diagnosis and treatment of DR is important because substantial and permanent vision loss may occur if DR is left untreated for 1 year or longer [3, 47]. An estimated 50,000 new cases of retinal neovascularization and diabetic macular edema (DME) occur yearly [120, 130], and as many as half of the patients who would benefit from treatment remain untreated [87].
Preventable causes of blindness such as cataracts, glaucoma, and infectious diseases are viewed as the most important ophthalmic public health problems in many parts of the world, making screening for diabetic retinopathy and subsequent treatment lesser priorities (Fig. 1.3). Fortunately, the incidence rate of nonproliferative diabetic retinopathy appears to be declining in the United States, thus supporting the contention that more aggressive management of DM and its comorbidities limits the development of DR-related vision loss. In response to the results of randomized, controlled trials, the initiation of the VISION 2020 program in 1999 [54, 172] and DR surveillance programs [141] has intensified control of risk factors [38, 143, 156], and continuing improvements in healthcare systems have contributed to the decreasing rates of DR [81]. Unfortunately, studies from less developed areas of the world have been more limited in scope and do not show similar trends [148].
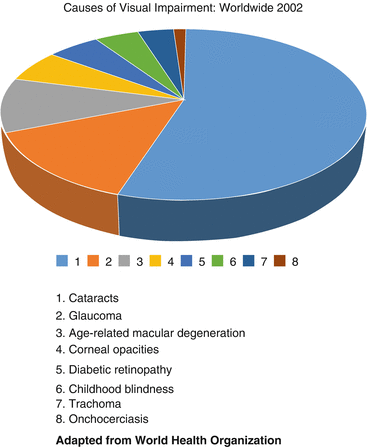

Fig. 1.3
This pie chart shows the most common causes of blindness throughout the world in 2002. Cataracts account for most cases of blindness, though these are almost exclusively from developing countries and reflect an inadequate supply of the healthcare services that are abundant in industrialized countries. Diabetic retinopathy accounts for about 5% of worldwide blindness (fifth on the list) and is a major cause of blindness in industrialized countries
The number of people worldwide with DR will increase from 126.6 million in 2010 to 191.0 million by 2030, and the number with vision-threatening diabetic retinopathy (VTDR) is estimated to increase from 37.3 million to 56.3 million [183]. Among patients aged 20–79 years with diabetes, the worldwide prevalence of any grade of DR has been estimated to be 35% and that of PDR to be 7% [80]. Among patients with DM for at least 25 years, 50% of those with T1DM and 15% of those with T2DM will have proliferative retinopathy [6].
A pooled analysis using individual participant data (22,896 individuals) from 35 population-based studies (1980–2008) from around the world was performed to determine the prevalence of DR [177]. The overall prevalence of DR was 34.6% (95% CI 34.5–34.8%) for any DR, 6.96% (CI 6.87–7.04%) for proliferative DR, 6.81% (CI 6.74–6.89%) for diabetic macular edema, and 10.2% (10.1–10.3%) for VTDR. All DR prevalence endpoints increased with diabetes duration, hemoglobin A1c levels, and blood pressure measurements, and were higher in people with T1DM compared with T2DM. The prevalence estimates of any DR and VTDR were similar in men and women and were highest in African-Americans and lowest in Asians [146, 176]. Higher total serum cholesterol was associated with a higher prevalence of DME, bringing clarity to previously conflicting reports about this risk factor [167]. The authors concluded that there are approximately 93 million people with DR, 17 million with proliferative DR, 21 million with diabetic macular edema, and 28 million with VTDR worldwide.
An analysis of pooled data from several population-based studies estimated that approximately 40% of patients with diabetes who are over the age of 40 years have some retinopathy and 8.2% have vision-threatening retinopathy [81].
Many have attempted to define the contribution of hereditary factors to the development of DR [144], but no firm data suggests that DR has a genetic component. This differs from diabetic nephropathy where important genetic associations have been described recently [43]. Candidate gene studies and GWAS (genome-wide association studies) may ultimately find genetic linkage to retinopathy phenotypes.
The WHO (World Health Organization) has estimated that diabetic retinopathy accounts for approximately 15–17% of total blindness in Europe and the United States [134]. The widespread use of drugs that inhibit the actions of vascular endothelial growth factor (VEGF) has significantly decreased the incidence of blindness due to age-related macular degeneration (AMD), prompting some authors to speculate that diabetes has become the overall leading cause of blindness [28, 45]. Further complicating the care of many of these patients is the fact that diabetes is also a leading cause of renal failure through its effects on the microvasculature [29, 77, 126]. Since prevalence rates of systemic arterial hypertension – a well-established risk factor for DR – are also increasing, the importance of DR to vision loss in industrialized nations is unlikely to diminish [70, 131].
Diabetic retinopathy is responsible for up to 17% of all blindness in parts of the Americas, Europe, and the Western Pacific [163]. The numbers of patients with both DM and DR appear to be increasing, and by 2050 as many as 50 million or more individuals in the United States will have DM with half having some form of retinopathy [10, 18, 19, 75, 81, 116]. In 2004, the Eye Diseases Prevalence Research Group estimated the prevalence of diabetic retinopathy from the compilation of eight separate population-based studies from the United States and elsewhere that had been conducted in the late 1980s or early 1990s [81]. Their report recommended that more recent estimates of diabetic retinopathy prevalence be obtained from the nationally representative sample of the National Health and Nutrition Examination Survey (NHANES) [181].
The 2005–2008 National Health and Nutrition Examination Survey evaluated 5222 Americans aged 40 years and over for general visual impairment (distance visual acuity worse than 20/40 in the better-seeing eye) and visual impairment not due to refractive error (distance visual acuity worse than 20/40 after refraction). The overall prevalences of visual impairment and of visual impairment not due to refractive error were 7.5% (95% CI, 6.9%, 8.1%) and 2.0% (95% CI, 1.7%, 2.3%), respectively. Not surprisingly, the prevalence of visual impairment not due to refractive error was significantly higher among people with DR (3.5%) compared to those without DR (1.2%) [28]. The prevalences of DR and vision-threatening DR were found to be 28.5% (95% CI, 24.9–32.5%) and 4.4% (95% CI, 3.5–5.7%). A slightly greater prevalence of DR was found among men (31.6%, 95% CI, 26.8–36.8%) than women (25.7%, 95% CI, 21.7–30.1%, P = 0.04). Non-Hispanic blacks, compared to non-Hispanic whites, had higher incidences of DR (38.8%, 95% CI, 31.9–46.1% vs. 26.4%, 95% CI, 21.4–32.2%, P = 0.01) and vision-threatening retinopathy (9.3%, 95% CI, 5.9–14.4% vs. 3.2%, 95% CI, 2.0–5.1%, P = 0.01). Male gender was independently associated with the presence of DR (OR 2.07, 95% CI 1.39–3.10), higher HbA1c (OR, 1.45, 95% CI, 1.20–1.75), longer duration of diabetes (OR, 1.06 per year duration, 95% CI, 1.03–1.10), insulin use (OR, 3.23, 95% CI, 1.03–1.10), and higher systolic blood pressure (OR, 1.03 per mmHg, 95% CI, 1.02–1.03).
A previous analysis of NHANES III data suggests that the prevalence of diabetic retinopathy is 46% higher in non-Hispanic black individuals and 84% higher in Mexican Americans than in non-Hispanic whites [61, 63]. The prevalence of vision-threatening diabetic retinopathy was 190% higher in non-Hispanic blacks and 130% higher in Mexican Americans than in non-Hispanic whites, probably because non-Hispanic blacks and Mexican Americans have poorer diabetes control and are less likely to be screened and treated in a timely manner [127]. Non-Hispanic black individuals and Hispanics are less likely to use eye care services [182]. These findings highlight the need to reduce disparities in care among racial, ethnic, and socioeconomic groups [182].
Latino Americans have one of the highest rates of visual impairment but the prevalence and risk of undetected eye disease cannot be accurately quantified. In one study, the 4-year incidences of best corrected visual impairment and blindness from DR in Latinos were 1.2 and 0.3%, respectively [159]. Access to healthcare by this population is inconsistent and unequal compared to other populations, and this may influence disease statistics. In contrast, no association was found between socioeconomic status and DR in cohorts of Mexican Americans and Caucasian patients with T2DM in Texas [60]. Approximately 13% of African-Americans have T2D, with the prevalence and incidence of T2DM being at least twice as high as that among white Americans [18, 62].
There are more than two million Native Americans on the North American continent, comprising more than 500 tribal organizations, and a comprehensive review of complications of T2DM in this indigenous population reveals high prevalence rates of DR for all populations studied. High prevalence rates of DR have been observed among the Alberta First Nations of Canada (40%) and the Pima Indians in Arizona (37.8%). In the Southern Alberta Study of Diabetic Retinopathy, DR in nonnatives tended to be more advanced, but the prevalences of DR in native and nonnative Canadians were identical (40%).
The Eye Disease Prevalence Research Group estimated that 4.1 million Americans had diabetic retinopathy in 2010 and projected that this would rise to 7.2 million by 2020 [118]. It has been estimated that one in every 12 Americans with diabetes over the age of 39 has vision-threatening retinopathy. A recently published study of the prevalence of DR and vision-threatening DR in a nationally representative sample of US adults aged 40 years or older showed that approximately 1.5% (95% CI, 1.1–2.2%) of adults with diabetes had proliferative DR and 2.7% (95% CI, 1.8–4.0%) had CSME [181]. Over a 10-year period, nonclinically significant DME and CSME will develop in 14% and 10%, respectively, of Americans with known diabetes [89]. Approximately half of all patients with DME will lose two lines or more of vision within 2 years [47, 48].
A cross-sectional, subgroup analysis of fundus photographs from 1038 participants with diabetes aged 40 years or older showed that 55 had DME, for an overall weighted prevalence of 3.8% (95% CI, 2.7–4.9%); this translates to 746,000 persons with DME in the US 2010 population. There were no differences in prevalence rates due to age or gender, but the chances of having DME were higher for non-Hispanic blacks than for non-Hispanic whites (odds ratio [OR], 2.64). Elevated hemoglobin A1c levels (OR, 1.47; P < .001) and longer duration of diabetes (OR, 8.51; P < .001) were also associated with DME [13].
Diabetic retinopathy appears to be strongly associated with all-cause mortality. An analysis of 20 studies with data from 19,234 patients showed that any degree of DR increased the risk of all-cause mortality by 2.34-fold (95% CI, 1.96–2.80) among type 2 diabetics and 2.41-fold (95% CI, 1.87–3.10) among type 1 diabetics [95]. Fortunately, however, rates of non-ocular diabetic complications have declined during recent decades. The hospitalization rate for lower extremity amputations among individuals with diabetes began decreasing in 1997 [57], and the prevalence of diabetes-related end-stage renal disease decreased between 1996 and 2006 [16]. It is reasonable to assume from these favorable trends in incidence data that improved diabetes care, such as effective management of blood glucose levels, blood pressure, and serum lipid levels, may also be reducing the incidence of diabetic retinopathy [16, 57].
Population-based studies have shown that nearly all patients with T1DM and more than 60% of those with T2DM develop DR during the first two decades of the disease [51]. Recent population-based studies have reported decreases in the prevalence and incidence of severe DR [67, 92, 106]. These findings, however, were limited to regional populations, and broad application of this data to national populations cannot be done with certainty [181]. The prevalence of DR has been changing because of improvements in management of blood glucose, serum lipids, and blood pressure [4]. In the 8 years between the beginning of the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) and the beginning of the Beaver Dam Eye Study (BDES), the prevalence of any DR in persons with T2DM fell by 30% (from 50% in the WESDR in 1980–1982 to 35% in the BDES in 1988–1990), and the prevalence of vision-threatening DR fell by 70% (from 10% in the WESDR in 1980–1982 to 3% in the BDES in 1988–1990) [85, 86, 88].
The Wisconsin Epidemiologic Study of Diabetic Retinopathy reported that the 10-year incidence of DME in the United States was 20.1% among type 1 diabetics, 25.4% among insulin-dependent type 2 diabetics, and 13.9% for non-insulin-dependent type 2 diabetics. The prevalence of DME was 3% (diabetes duration, 0–14 years) or 6% (>15 years) in mild NPDR, 37% (0–14 years) or 63% (>15 years) in moderate/severe NPDR, and 73% (0–14 years) or 74% (>15 years) in PDR [82]. Nearly half of the patients with DME will lose two or more lines of visual acuity within 2 years.
National DR screening programs have been introduced to all four countries of the United Kingdom during the last decade resulting in significant reorganization of care for these patients [17, 124, 135, 141]. Analysis of a large UK database found that 15.8–18.1% of diabetics had center-involving macula edema [80]. Of these, 14% of eyes had mild NPDR, and 24% of eyes with moderate NPDR, 31% of eyes with severe NPDR, and 22% of eyes with PDR also had CSME (whether or not center-involving). Prevalence figures for DR are approximately three times higher in the hospital databases than whole population estimates, reflecting the tendency that patients with more advanced grades of DR are managed in the hospital systems. The prevalence of any grade of DR was around 80% in this study, compared with 20–40% in most population studies of patients with diabetes. PDR prevalence was around 20%, and CSME prevalence was around 18% in the hospital database, compared with 1–8% and 6%, respectively, in the general populations [14, 107, 111, 133, 150, 178].
From the English National Health System database, the number of people with diabetes in England in 2010 was estimated at 2,342,951 of which 2,334,550 were over the age of 12 years. An estimated 166,325 (7.12%) had DME in one or both eyes, and of these, 64,725 individuals had DME that reduced the visual acuity to poorer than 6/6 in at least one eye. The overall health and social care costs in 2010, from screening through treatment, rehabilitation, and care in the home, were estimated at £116,296,038.
The community-based National Diabetic Retinopathy Screening Service for Wales performed a cross-sectional analysis of 91,393 persons with diabetes (5003 with T1DM and 86,390 with T2DM) at their first screening from 2005 to 2009. The prevalence of any DR and sight-threatening DR in patients with T1DM was 56.0% and 11.2%, respectively, and in patients with T2DM was 30.3% and 2.9%, respectively. The presence of DR was strongly associated with increasing duration of diabetes for patients with either T1DM or T2DM and was also associated with insulin therapy in patients with T2DM.
A UK database study evaluated the incidence of DR in newly diagnosed diabetics [100]. By 9 years after the diagnosis of diabetes, 28% of T2DM and 24% of T1DM patients had developed DR (7899 incident DR cases). During the first 2 years with diabetes, the incidence rate was almost two times higher in patients diagnosed with diabetes in 2006–2007 than among those diagnosed in 2000–2001. Among patients with retinopathy at baseline, the study found a cumulative incidence of DME in 12.1% of T2DM patients and 18.8% of T1DM patients within 9 years.
The World Health Organization estimates that over 40 million Chinese will have diabetes by 2030 [164]. Similar to the studies in South Asia, the Beijing Eye Study reported that DR is a relatively minor cause of blindness (7.7%) in the Chinese population [78]. Nonetheless, with continued economic progress, this is becoming a threat to public health in many areas. In China, the prevalence of diabetic retinopathy among people with diabetes is predicted to reach 43% [177], with up to 9.2 million people in rural areas having diabetic retinopathy and 1.3 million having sight-threatening diabetic retinopathy. Reflecting the fact that DM and DR are related to lifestyle, DR is more prevalent in South Asians living outside the Indian subcontinent [138] and in urban cities [137] compared to those who reside in rural areas within India [115]. Projections suggest that for India alone, 0.7 million people will have proliferative diabetic retinopathy (PDR) in 2030, and 1.8 million will have clinically significant macular edema [139].
A Japanese study found a significant correlation between the severity of DME and the DR grade [175]. In this report, 28% of patients with mild/moderate NPDR, 67% with severe NPDR, and 51% with PDR also had DME.
Studies from Australia that date back more than three decades support the impact of health education and better glycemic control on the prevalence and incidence of DR [35, 104, 114]. In the past three decades, the prevalence and incidence of DR among patients with T1DM have declined in the United States, Australia, and other developed countries [183]. The earliest clinic-based study of DR in Australia, the Newcastle Diabetic Retinopathy Study (1977–1988), reported a 35% prevalence of DR, but subsequent population-based studies have reported lower rates [109] with the Australian Diabetes, Obesity, and Lifestyle study (AusDiab) reporting a DR prevalence of only 21.9%.
Comparing the prevalence rates for DR among studies is difficult because of the changing classification of diabetes over time, the different grading protocols employed, and differences in the characteristics of “similar” populations [177, 178, 181]. Findings from these studies, however, can provide policy makers with important information to plan eye care services, with the understanding that the prevalence of sight-threatening DR may be underestimated. The strong association between duration of diabetes and risk of retinopathy underscores the importance of early detection via office-based examinations or screening programs. An effectively structured screening program may reduce the incidence of blindness by 40% within 4 years [121].
Improving glycemic control by lowering the level of glycosylated hemoglobin (HbA1c) is the most effective way to slow the progression of DR. Keys to the discovery that optimal metabolic control could reduce the incidence and progression of DR were the DCCT and the UKPDS trials. Not only did intensive glycemic control inhibit the development of DR, these effects persisted well beyond the course of treatment [39, 40].
The Diabetes Control and Complications Trial (DCCT) (1982–1993) was a multicenter, controlled clinical trial that compared the effects of intensive blood glucose control (INT) with standard control (CON) on the onset and progression of DR. The Epidemiology of Diabetes Interventions and Complications (EDIC) study (1994–present) is an observational follow-up of the DCCT cohort. Of the 1441 DCCT subjects, 726 had no DR (primary prevention cohort) and 715 had mild DR (secondary intervention cohort) at baseline. Subjects were followed for a mean of 6.5 years. The median HbA1c was 7% in the INT group compared with a median of 9% in the CON group. INT reduced the adjusted mean risk for the development of DR by 76% and slowed progression of DR by 54% compared with CON.
Following DCCT, the HbA1c levels in the original INT and CON groups converged (year 8, INT 7.98%, CON 8.07%), but the INT group continued to enjoy a durable effect with significantly lower incidence of further DR progression (hazard reduction, 53–56%). Serious retinal outcomes and the need for procedures to treat them were reduced by 50% in the original INT group.
DCCT demonstrated that intensive glucose-lowering therapy for a mean of 6.5 years reduces the risk of development and progression of retinopathy by as much as 76% compared with conventional therapy. Much of the original effect persisted for over 18 years of follow-up in EDIC – the so-called metabolic memory – as the cumulative incidence of each retinal outcome continued to be lower in the former INT group. Interestingly, the year-to-year incidence of these outcomes is now similar in the two groups because of a reduction in risk in the former CON group [40].
1.4 Comorbidities for Diabetes and Diabetic Retinopathy
Higher levels of hemoglobin A1c, longer duration of diabetes, insulin use, and higher systolic blood pressure have been independently found to be associated with diabetic retinopathy [85, 86, 151, 165, 166, 182]. The early randomized controlled clinical trials showed that modifying risk factors such as hyperglycemia and blood pressure could reduce the burden of diabetic retinopathy and prevent vision loss [38, 155, 156] (Table 1.1). Efforts to prevent the development and progression of DR should target individuals with the highest risk of developing retinopathy, those with longer duration of diabetes, and those using insulin. Improved availability of care for vulnerable sections of the population should be able to reduce the risk of blindness in diabetes. Identifying and protecting individuals at risk of T2DM, by reducing body weight and increasing physical activity, may also help delay the onset of T2DM and reduce complications of diabetic retinopathy.
Table 1.1
This table lists some of the important trials that have studied the effects of the major systemic contributing factors (hyperglycemia, systemic arterial hypertension, hyperlipidemia) on the development and progression of diabetic retinopathy
Important trials associating diabetic retinopathy with major systemic risk factors | |
|---|---|
Hyperglycemia | |
Diabetes Complications and Control Trials (DCCT) Type 1 diabetes mellitus | Intensive glucose control: 1. Reduced the adjusted mean risk for the development of DR by 76% 2. Slowed progression of DR by 54% |
UK Prospective Diabetes Study (UKPDS) Type 2 diabetes mellitus | Intensive glucose control: 1. Reduced progression by 35% per A1C point 2. Reduced moderate vision loss by 47% |
Systemic arterial hypertension | |
Wisconsin Epidemiologic Study of Diabetic Retinopathy | Progression of retinopathy was associated with: 1. Higher diastolic BP at baseline 2. Increase in diastolic BP over a 4-year period |
UK Prospective Diabetes Study (UKPDS) Type 2 diabetes mellitus | Found that systolic BP <150 mmHg: 1. Decreased the progression of DR 2. Decreased the need for macular laser photocoagulation for DME |
ACCORD-Eye trial | Found that intensive BP control did not decrease the progression of DR |
EUCLID study | Found that lisinopril decreased the progression of DR in normotensive type 1 diabetics |
Diabetic Retinopathy Candesartan Trials (DIRECT) | In type 1 diabetics, 5 years of treatment: 1. Decreased the incidence of DR 2. Had no effect on progression of established DR In type 2 diabetics, there was a 34% regression of DR (P = 0.009) Less severe retinopathy in types 1 and 2 (P = 0.03) |
RASS trial | Evaluated 285 normotensive patients treated with enalapril, losartan, or placebo for 5 yrs Progression of retinopathy by two steps in: Placebo (38%) Enalapril (25%, P = 0.02) Losartan (21%, P = 0.008) Enalapril and losartan increased the likelihood of less DR progression by 65% and 70% independent of blood pressure lowering |
Hyperlipidemia | |
Fenofibrate Intervention and Event Lowering in Diabetes Study (FIELD) | Found that fenofibrate: 1. Decreased the requirement for the first laser and the development of DME 2. Decreased the need for laser treatment compared to the control group (3.4% vs. 4.9%, P = 0.0002) 3. Appeared to have protective effects independent of blood glucose, blood pressure, and baseline lipid values |
ACCORD-Eye study | The addition of fenofibrate to basal statin therapy resulted in: 1. A decrease in the progression of DR, in a similar manner to that observed with intensifying blood glucose control, but with a good safety profile without increasing the risk of hypoglycemia 2. Questions regarding fenofibrate’s mechanism of action and the pathogenesis of DR/DME |
Diabetic retinopathy is associated with increased cardiovascular (CV) and all-cause mortality in patients with T2DM and T1DM [41, 84, 149, 158]. Diabetic retinopathy also predicts all-cause mortality, more than just CV events. Several mortality studies showed that CV disease was the cause of death in fewer than 35% of diabetic patients [61, 84], suggesting that an alternative mechanism increases the death rate in many DR patients. An autonomic neuropathy could link DR and CV events since it was recently demonstrated that autonomic deregulation can lead to alterations in blood pressure and cardiac rhythm and the development of DR [5, 94].
In a recent systematic review of 17 studies of 14,896 people with T2DM (mean age 58 years and mean follow-up 9 years), people with any retinopathy were more than twice as likely to die or suffer a fatal or nonfatal CV event than people without retinopathy, and a fourfold higher risk was noted for people with advanced retinopathy. The same review also included four studies of 4438 people with T1DM (mean age 33 years and mean follow-up 12 years) and reported a 3.5–4-fold higher risk of death as well as CV events in the presence of any retinopathy and a sevenfold higher risk with advanced retinopathy.
Several explanations may account for the relationship between retinopathy and CV outcomes. First, both retinopathy and incident CV outcomes are recognized consequences of diabetes. Second, the degree of retinopathy is progressively related to the degree of several independent risk factors of CV outcomes, including hyperglycemia, elevated blood pressure, albuminuria, renal insufficiency, hyperlipidemia, and other abnormalities. People with these risk factors would therefore have both more severe retinopathy and a higher incidence of CV outcomes. Third, the microvascular abnormalities present in the retina may also be occurring in many other vascular beds, and CV outcomes may be due in part to accumulated microvascular abnormalities in the myocardial microcirculation, arterial wall, and elsewhere. If this is true, changes in retinal pathology may closely reflect changes in microvascular pathology in these other vascular beds, and people with the most rapid progression in retinal pathology may be the ones most likely to suffer adverse CV events.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree