(1)
Mayo Clinic, Jacksonville, Florida, USA
9.1 Introduction
Current pharmacologic treatment of diabetic retinopathy (DR) is based on the intraocular administration of several medications. Injections into the vitreous have emerged as the preferred route of drug delivery for several reasons. Firstly, drug concentrations in the vitreous, retina, and choroid are higher after intravitreal injections than after any other route of administration. Secondly, the vitreous acts as a depot that slows drug elimination from the eye and prolongs its duration of action. Thirdly, the small volume of injected drug results in low serum concentrations and may minimize the likelihood of systemic adverse events.
The favorable safety profile attributed to intraocular pharmacotherapy has contributed to the rapid development and widespread adoption of corticosteroid formulations and drugs that bind vascular endothelial growth factor (VEGF). The pivotal phase III registration trials were sufficiently sized to demonstrate clinically efficacy and garner approval by the United States Food and Drug Administration (US FDA), but they were insufficiently powered to identify low-frequency ocular and systemic adverse events. Consequently, systematic reviews and meta-analyses have been performed to better understand the risks of therapy.
The safety profiles of intraocular corticosteroids differ significantly from those of the anti-VEGF drugs. The volume of administered corticosteroids is not sufficient to cause systemic adverse events, but steroid therapy is complicated by high rates of cataract development and glaucoma, and triamcinolone acetonide injections may cause a severe sterile endophthalmitis or pseudoendophthalmitis.
Compared to both the general population and other groups who receive intraocular pharmacotherapy, patients with diabetes may be at greater risk of developing thromboembolic events and impaired wound healing, which might be worsened by anti-VEGF therapy. Physicians, therefore, remain keenly interested in the ocular and systemic safety profiles of drugs used to treat DR. The aim of this chapter is to discuss known risks of drugs used to treat DR.
9.1.1 Intravitreal Injections
Drugs are injected into the mid-vitreous through the pars plana (3–4 mm posterior to the limbus) (Fig. 9.1). Vascular endothelial growth factor inhibitory drugs may be injected through 30- or 32-gauge needles, whereas triamcinolone acetonide can be injected through 27- or 30-gauge needles. Large volumes (1 ml) of triamcinolone suspension frequently occlude 30-gauge needles, but the author has never experienced occlusion when injecting small intravitreal volumes (0.05 ml). Needles may be safely inserted to a depth of 12 mm and should be directed toward the mid-vitreous. The bioerodible, sustained release dexamethasone phosphate insert (Ozurdex®, Allergan, Irvine, CA) is injected through a pre-loaded 22-gauge insertion system, whereas the non-bioerodible, sustained release fluocinolone acetonide insert (Iluvien®, Alimera, Alpharetta, GA) is injected through a pre-loaded 25-gauge insertion system.
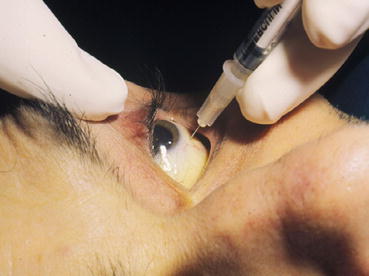

Fig. 9.1
This photograph of the author’s technique of performing an intravitreal injection shows the inferior insertion position of the needle as the patient looks up
Neither preinjection nor postinjection antibiotics appear to alter low postinjection endophthalmitis rates [10, 48], but practice patterns regarding antibiotic use differ widely among surgeons. Most physicians use an eyelid speculum to stabilize the eyelids and improve exposure of the eye, but excellent results have been reported by having the surgeon or an assistant manually retract the eyelids (Fig. 9.2) [68]. Topical povidone-iodine, either 5% or 10%, should be used to sterilize the conjunctiva prior to the injection [17, 66]. True allergy to povidone-iodine is rare and most adverse reactions are due to a toxic keratitis. Patients who claim to be “allergic to iodine” because of reactions to shellfish (allergy to myosin) or intravenous contrast dye (hyperosmotic reaction) can be assured that there is no relationship between these adverse reactions and either iodine or povidone-iodine.
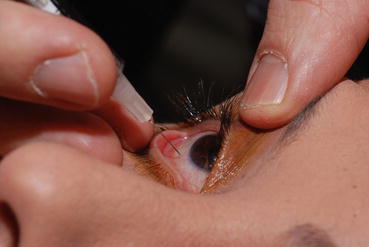

Fig. 9.2
This photograph of the author’s technique for performing an intravitreal injection shows the manual retraction of the eyelids without the use of an eyelid speculum or surgical assistant
Various forms of anesthesia can be administered – topical proparacaine or tetracaine drops, lidocaine gel, subconjunctival lidocaine injection, or peribulbar lidocaine injection – to decrease the pain experienced by patients. Each form of anesthesia has its associated advantages and drawbacks. For example, topical drops provide less predictable anesthesia but allows the procedure to be performed very quickly; subconjunctival lidocaine improves the depth of the anesthesia but prolongs the procedure and causes a subconjunctival hemorrhage in more than 50% of cases. The overall experience with the procedure appears to be independent of the method of anesthesia [11]. Surgeons, therefore, should find a technique with which they are most comfortable but should always consider individualizing anesthesia to meet the needs of each patient. Since the needles on the dexamethasone (22 gauge) and fluocinolone (25 gauge) insertion devices have considerably larger bores than those usually used for anti-VEGF injections (30 gauge), surgeons should consider the routine use of subconjunctival lidocaine when injecting these steroids.
Injections may be safely and efficiently performed in an outpatient clinic or, as is required in some European countries, within an operating suite. Some physicians contend that the incidence of endophthalmitis may be higher when injections are performed in the clinic rather than in the operating room [1], but the results from large pooled series of injections do not support this. Some clinics deal with the increasing number of intravitreal injections by employing specially trained nurses. In one New Zealand clinic, nurse specialists administered a total of 2900 injections over an 18-month period. Two patients (0.07%) developed postinjection endophthalmitis (1 microbial and 1 nonmicrobial), two patients (0.07%) developed vitreous hemorrhages, and five patients (0.17%) had elevated intraocular pressures (IOPs) [60]. The anticipated growth of intravitreal pharmacotherapy will likely result in an increase in the use of physician extenders.
9.2 Ocular Complications
Tables 9.1 and 9.2 list the complication rates of ocular adverse events due to anti-VEGF drugs and corticosteroids, respectively.
Table 9.1
The incidences of ocular adverse events that have been described in patients receiving treatment with vascular endothelial growth factor inhibitors
Ocular adverse events due to anti-VEGF drugs | |
|---|---|
Complication | Incidence rate |
Increased intraocular pressure | 3–12% |
Cataracts | <1% to 3.7% |
Endophthalmitis | From: 0.02% per injection to 1%/patient over 3 years |
Sterile inflammation | 0–1.49%/injection |
Retinal detachment | 0.9%/injection to 3% per eye *most may be exacerbation of traction detachment |
Vitreous hemorrhage | 0.4% |
Worsening traction detachment | 5.2% |
Table 9.2
The incidences of ocular adverse events that have been described in patients receiving treatment with intravitreal corticosteroids
Ocular adverse events due to corticosteroids | |
|---|---|
Complication | Incidence rate |
Cataracts | 37–80% |
Glaucoma | 32–40% |
Endophthalmitis | 0.03–0.09% per Injection |
Migration of dexamethasone insert into anterior chamber | |
9.3 Elevated Intraocular Pressure (IOP) and Glaucoma
Population studies have defined the normal human IOP (encompassing 95% of the population) as 9–22 mmHg. Intraocular pressure is maintained through a complex balance of aqueous production, ocular rigidity, tissue deturgescence, and aqueous outflow. Most studies do not implicate diabetes as a risk factor for glaucoma, but intravitreal pharmacotherapy increases the risk of glaucoma in this population. In patients with diabetic macular edema (DME) that receive pharmacotherapy, elevated IOP usually results from increased resistance to aqueous outflow. Since eyes of diabetic patients frequently have complex vitreoretinal pathologies, IOP elevations should prompt evaluations for causes of secondary open-angle (corticosteroid-induced, anti-VEGF-induced, hemolytic, ghost-cell) and secondary angle-closure (neovascular) glaucoma.
The evaluation and treatment of glaucoma encompasses an entire subspecialty within ophthalmology that is beyond the scope of this book. Physicians who manage DME, however, need to be continually aware of the IOP and health of the optic nerve and nerve fiber layer. Intraocular pressure should be measured at each visit, and the status of the optic nerve and nerve fiber layer should be assessed periodically with disc photographs and/or optical coherence tomography (OCT) measurements. Any IOP elevation should prompt the physician to consider testing (assessment of optic disc anatomy with biomicroscopy and stereoscopic disc photographs, OCT analysis of the nerve fiber layer, threshold visual fields, and pachymetry). If treatment of prolonged IOP elevation is outside the scope of practice of the treating physician, then referral to a glaucoma subspecialist or qualified comprehensive ophthalmologist should be considered.
Intraocular pressure often rises immediately after intravitreal injections but usually normalizes quickly. One study demonstrated that >95% of eyes had an IOP <35 mmHg immediately after injection and only 33% had an IOP elevation of 10 mmHg [8]. Sustained elevations in intraocular pressure occur in approximately 6% of eyes receiving anti-VEGF injections [30] and after a median of five injections. Preexisting glaucoma is a risk factor for sustained IOP elevation, probably because of an already compromised aqueous outflow. Patients receiving at least 29 injections have a 5.75 times risk of developing sustained IOP elevation compared to those receiving 12 or fewer injections [33]. Reasons for a higher risk with more injections may include prolonged VEGF blockade, development of an inflammatory trabeculitis, impaired aqueous outflow due to protein aggregates in the trabecular meshwork, large macromolecules obstructing the meshwork because of bulk deposition, or silicone droplets from the needles and syringes.
An exploratory post hoc analysis of DRCR.net Protocol I sought to determine the incidence of elevated IOP in eyes without preexisting glaucoma. During the 3-year trial, sustained IOP elevation was seen in 6 patients in the laser/sham group and 22 patients receiving ranibizumab (Lucentis®, Genentech, S. San Francisco, CA/Roche, Basel, Switzerland). The risk of developing a sustained IOP of >22 mmHg with an elevation of ≥6 mmHg above baseline was 10% in patients receiving ranibizumab, but only 3% in patients randomized to laser/sham (hazard ratio 2.9 [95% CI, 1.0–7.9]). Through 1 year in the DRCR.net Protocol T trial, patients treated with aflibercept (Eylea®, Regeneron, Tarrytown, NY), bevacizumab (Avastin®, Genentech, S. San Francisco, CA/Roche, Basel, Switzerland), and ranibizumab had the following incidences of elevated intraocular pressures: 12%, 9%, and 9% [20].
In a large retrospective study of 760 eyes, both transient (7%) and sustained (5.8%) elevations of intraocular pressure (defined as increases ≥6 mmHg, >20% above baseline, or >24 mmHg on two or more consecutive visits) were seen after treatment. The probability of an IOP rise increased with the number of injections.
Development of glaucoma is a greater concern in patients receiving intraocular corticosteroids. Patients susceptible to steroid-induced glaucoma are believed to have upregulation of corticosteroid receptors in trabecular meshwork cells [81], which leads to increased expression of the extracellular protein fibronectin, glycosaminoglycans, and elastin [37, 67]. Steroids also downregulate phagocytic activity, which allows obstructive material to accumulate in the meshwork [58, 59]. Increased pressure in patients receiving triamcinolone acetonide may be due to obstruction of aqueous outflow due to steroid deposits in the angle. Approximately 50% of patients receiving intravitreal triamcinolone will experience an elevation in pressure within 2 to 4 weeks [64].
In the DRCR.net trial comparing macular laser photocoagulation with 1 mg and 4 mg intravitreal triamcinolone acetonide, the incidences of IOP elevation in the 1 mg and 4 mg triamcinolone cohorts were 20% and 40%, respectively [19]. Incisional glaucoma surgery was eventually required in 1.6% of patients receiving 4 mg triamcinolone. In DRCR.net Protocol I trial, 42% of patients receiving 4 mg triamcinolone experienced elevated IOP [22].
Intraocular pressure increases with the dexamethasone insert can generally be managed with pressure-lowering medications and are usually transient [9, 16, 31]. In a report of 186 eyes that received the dexamethasone insert through 6 months, eight eyes (4.3%) had intraocular pressure increases to at least 30 mmHg and one eye had an IOP of 50 mmHg [51]. The elevated pressure was successfully treated in all eyes with medications. In the phase III MEAD trials, patients receiving the 0.7 mg dexamethasone insert had a 32% incidence of IOP >25 mmHg and a 6% incidence of IOP >35 mmHg [12]. Less than 1% of eyes required incisional glaucoma surgery.
In the 36-month FAME trial, 38.4% of patients receiving the 0.2 mg fluocinolone insert (Iluvien) developed increased intraocular pressure, and 18.4% had IOPs over 30 mmHg [14]. Approximately 4% of patients required incisional glaucoma surgery to control the pressure. In a series of 17 eyes that were treated with the fluocinolone insert after having previously responded poorly to anti-VEGF therapy, only three had elevated IOP by 12 months and all were successfully treated with topical therapy [46].
9.4 Cataracts
Cataracts due to intravitreal injections of anti-VEGF drugs are very unusual. Only 1 injection-related cataract occurred after 6000 injections in DRCR.net Protocol T [20], presumably from needle trauma to the crystalline lens capsule (Fig. 9.3).
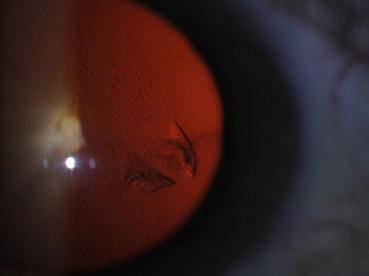

Fig. 9.3
This slit-lamp photograph shows a perforation of the posterior lens capsule following the intravitreal injection of a vascular endothelial growth factor inhibitory drug. Localized posterior subcapsular lens opacifications have developed
Corticosteroids induce posterior subcapsular cataracts (Fig. 9.4) because upregulated fibroblast growth factor-1, epidermal growth factor, transforming growth factor-β, lens epithelium-derived growth factor, platelet-derived growth factor, and bone morphogenic proteins induce proliferation and migration of lens epithelial cells. Local activation of glucocorticoid receptors in the lens may also downregulate apoptosis [35, 36].
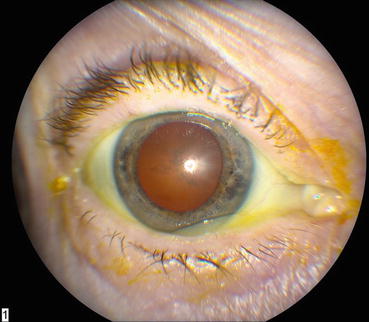

Fig. 9.4
This slit-lamp photograph shows a posterior subcapsular cataract that formed after treatment with intravitreal corticosteroids
Prospective studies evaluating the treatment of DME with triamcinolone acetonide report that the incidence of cataracts appears to be dose-related [19] and 37–54% of eyes undergo cataract surgery within 2 years [19, 28]. In the Protocol I trial, 15% of patients randomized to triamcinolone required cataract surgery within the first year compared to 3.7% of patients receiving ranibizumab and 6% of patients randomized to sham/laser [22]. The incidence of cataract surgery in the triamcinolone group increased to 55% at 2 years [23].
In the cohort of phakic eyes receiving the 0.7 mg dexamethasone insert in the phase III MEAD trials, progression of cataracts was experienced by 67.9% of eyes over 3 years [12]. In another dexamethasone insert study, progression of lens opacities was seen in 26 of 112 (23.2%) phakic patients within 6 months and 7 (6.3%) underwent cataract extraction [51]. This rate was higher than had been reported in other studies [9, 16, 27, 31].
9.5 Endophthalmitis
Individual cases and clusters of patients with noninfectious inflammation that followed injections of each anti-VEGF drug have been reported. In a study of 61 eyes with AMD, DME, or RVO, increased aqueous flare was detected in eyes that received bevacizumab but not ranibizumab or aflibercept (P = 0.006) [11]. Rates of inflammation after bevacizumab range from 0.14% to 0.32% and have been attributed to bevacizumab’s large size and Fc fragment [24, 26]. A review of postinjection inflammation reported rates of 0.09–1.49% with bevacizumab, 0–0.83% with ranibizumab, and 0–0.05% with aflibercept [3].
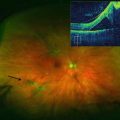
A large meta-analysis of intravitreal injections found that Staphylococcus epidermidis was the most common cause of infectious endophthalmitis [47], but a single-center report of over 65,000 injections reported that Streptococci was the most common organism (Fig. 9.5) [52]. Since most Streptococci originate from the oropharynx, some surgeons have begun wearing masks while injecting or minimizing conversation to minimize aerosol spread of organisms.
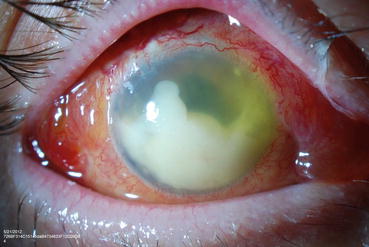

Fig. 9.5
This external photograph shows a large hypopyon in an eye with streptococcal endophthalmitis
The single-injection rates of endophthalmitis in the phase II and III DME trials were low, but the cumulative per patient endophthalmitis rates in the RESOLVE [45] and DRCR.net Protocol I [23] trials were approximately 1%. On the other hand, no cases of infectious endophthalmitis were reported in either the phase III RESTORE trial, which followed RESOLVE [62], or the DRCR.net Protocol T trial. Two cases of noninfectious inflammation occurred in each drug group (out of approximately 2000 injections with each drug) in Protocol T [20].
In some countries surgeons compound several doses of bevacizumab from the same vial to use in different patients during a single day. This may be done inside or outside of an operating suite. In one study, surgeons aliquoted bevacizumab from six vials and stored the syringes for up to 1 week at 4° C [18]. Subsequent analysis demonstrated no evidence of bacterial contamination and no cases of endophthalmitis occurred after 973 injections. The authors concluded that this technique is safe if “standard precautions” are adhered to. A retrospective report described a technique in which up to ten doses of bevacizumab were prepared by the surgeon from each vial [53], and no cases of endophthalmitis occurred after 1184 injections. Though this compounding procedure may be performed commonly in some areas, it does not comply with United States Pharmacopeia Chapter 797 guidelines and should be viewed with skepticism [29].
Bilateral same-day injections are performed by some surgeons and patients favor this technique since it decreases the number of office visits. In a series of 342 bilateral injections, one patient developed infectious endophthalmitis in only one eye. The authors claim that this technique is safe if the surgeon changes povidone-iodine, eyelid speculums, and all needles and syringes between injecting the two eyes [2].
Because bevacizumab must be compounded or fractionated from 4 ml multiuse vials into 20 to 30 single-dose 1 ml syringes, it may cause clusters of infectious endophthalmitis because of compounding errors. Several outbreaks of compounding-related endophthalmitis due to highly virulent organisms have occurred [29]. Visual outcomes usually depend upon the organism, but severe vision loss is common. Compounding is best performed by a trained technician working under a high-quality, laminar flow hood. By following the guidelines in Chapter 797 of the United States Pharmacopeia, these technicians are able to safely and inexpensively compound bevacizumab for intraocular use.
The overall incidence of endophthalmitis following intravitreal triamcinolone injections has been estimated at 0.8% for noninfectious causes and 0.6% from infectious agents [63]. The DRCR.net Protocol B trial reported only one case of endophthalmitis in 3159 triamcinolone injections (0.03% per injection) [19] though smaller series have reported higher incidence rates primarily because of the smaller numbers of injections. The cause of sterile or noninfectious endophthalmitis is unknown, but some investigators believe that it may be due to preservatives. Pseudoendophthalmitis occurs when the crystalline triamcinolone suspension diffuses rapidly throughout an eye that has previously undergone vitrectomy; fortunately this is a self-limited condition.
The incidence of endophthalmitis following insertion of the dexamethasone insert appears to be low. In the MEAD trial, 2928 DEX injections were performed, with only two cases of endophthalmitis [12]. One of these occurred after cataract surgery and was felt to be unrelated to the DEX.
9.6 Counterfeit Drugs
Bevacizumab ranks seventh in world drug sales (by dollars) with most of this stemming from oncologic use [56]. Because bevacizumab is manufactured as a clear liquid, it has become an easy target for counterfeiters. Counterfeit bevacizumab naturally lacks biological efficacy and the substitute solution may be contaminated with microbes or pro-inflammatory substances. Though intravenous injections of counterfeit bevacizumab may not cause observable adverse events, intraocular injections have caused both infectious endophthalmitis and severe sterile inflammation with retinal necrosis.
Series of patients receiving intravitreal injections of counterfeit bevacizumab have been reported in China [75], Mexico [25], and India [69]. Counterfeit bevacizumab has entered the supply chain in the United States though no injuries were reported [76].
Bevacizumab should always be purchased from a reputable supplier, and physicians and pharmacists must carefully scrutinize bottles for authenticity to be sure that they are not using counterfeit products.
9.7 Other Complications
Retinal detachments (RDs) are frequently cited as complications of intravitreal injections, but they occur only rarely. A systematic review put the RD rate at 3.9% per eye and 0.9% per injection [34], but most of these detachments were believed due to vitreoretinal traction from the underlying disorder rather than due to the injection procedure. Detachment rates from the phase III DME registration trials, from which eyes with significant fibrovascular proliferation were excluded, are generally much lower.
Intraocular hemorrhages (retinal, vitreous, or hyphema) have been reported after intraocular injections, but like RDs, these are mostly due to the underlying diabetic retinopathy. The incidence of vitreous hemorrhage in RISE and RIDE was only 0.4% in the treatment arms compared to 2.8% in the sham arm [54], suggesting that most hemorrhages were due to progression of the diabetic retinopathy. Subconjunctival hemorrhages are quite common (10%), particularly in patients taking aspirin (17%) [41]. Most studies do not report an increased incidence of intraocular hemorrhages in patients taking systemic anticoagulants [42, 44], and special precautions are not required when injections are performed in these patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree