(1)
Mayo Clinic, Jacksonville, Florida, USA
10.1 Introduction
Diabetes mellitus (DM) significantly impacts quality of life [8, 16, 48] with affected patients unable to manage their diabetes as well when they are visually impaired [6]. Vision impairment compromises quality of life by limiting physical activity, promoting social isolation, and causing dependence on others for the performance of many of life’s necessary functions [43]. Patients with diabetic macular edema (DME) also have higher rates of healthcare utilization (doctors’ visits, hospitalizations, diagnostic testing, treatments, and medications) compared to diabetic patients without diabetic retinopathy [20, 26, 44]. A US health quality study reported that diabetes is associated with a utility of 0.53, whereas blindness has a utility of only 0.38, with only major stroke (0.31) and end-stage renal disease (0.35) ranking lower [19]. By identifying patients with diabetic retinopathy (DR), physicians are frequently able to stabilize and improve vision, thereby improving the quality of patients’ lives. Improvement in visual acuity often enables patients to resume driving and obtain gainful employment.
For decades, the standard treatment for most patients with vision-threatening DR was laser photocoagulation, with pars plana vitrectomy reserved for advanced vitreoretinal pathologies. As discussed in other chapters in this text, laser reduces the risk of vision loss by approximately 50% in most patients with DME and proliferative diabetic retinopathy, but does not usually improve visual acuity. Maintaining good visual acuity also depends upon early diagnosis through effective screening programs, regularly scheduled examinations, and prompt examination and treatment of symptomatic and at-risk patients.
Intravitreal pharmacotherapy with corticosteroids and drugs that inhibit the actions of vascular endothelial growth factor (VEGF) is capable of significantly improving vision in a majority of treated patients. Physicians in several countries have studied the cost-effectiveness of diabetic retinopathy treatments, and their conclusions vary according to the drug costs in each country and the assumptions used to create each of the respective economic models. All generally agree, however, that treatment of DR with laser and pharmacotherapy is cost-effective. The cost of drugs in each country remains a major determinant of cost-effectiveness, and future models will have to contend with the major price differences between compounded bevacizumab and commercially provided ranibizumab and aflibercept. In some countries, this comparison is moot because intraocular injection of compounded bevacizumab is prohibited by regulatory authorities.
The costs associated with intravitreal pharmacotherapy can be considerable and they can impact private insurance companies and national health systems. This chapter examines the costs of therapy and the subsequent effects on insurance and healthcare policy.
10.2 National Costs
In the United States, diabetes accounts for 10% of healthcare spending and is the leading cause of new blindness in working aged adults [5]. Approximately 75,000 new cases of DME are diagnosed yearly and medical costs for these patients are 29% higher than for diabetic patients without DME. Inpatient care comprises nearly half of these costs [44].
The pharmaceutical costs associated with the treatment of DME in the United States are considerable. Insurance payments for intravitreal drugs administered to Medicare patients are paid through Part B. In 2010, when intravitreal pharmacotherapy was being used predominantly for the treatment of neovascular age-related degeneration, VEGF inhibitors accounted for $2 billion or one-sixth of the US Medicare Part B drug budget [49]. By 2013, Part B expenditures for ranibizumab and aflibercept totaled $2.5 billion [50]; during calendar year 2014, ranibizumab ($1.3 billion) and aflibercept ($1.3 billion) were the second and third most expensive drugs (behind rituximab) (Table 10.1). The majority of these payments were for the treatment of neovascular age-related macular degeneration, but an increasing proportion was for DME.
Table 10.1
The 2014 Medicare Part B expenditures for the top five drugs
Generic and brand names | Major indications | Total spending |
|---|---|---|
Rituximab (Rituxan) | Non-Hodgkin lymphoma, chronic lymphocytic leukemia | $1.4 Billion |
Ranibizumab (Lucentis) | Neovascular AMD, DME, edema due to RVOs | $1.3 Billion |
Aflibercept (Eylea) | Neovascular AMD, DME, edema due to RVOs | $1.3 Billion |
Infliximab (Remicade) | Rheumatoid arthritis, psoriasis, Crohn’s disease | $1.2 Billion |
Pegfilgrastim (Neulasta) | Myelosuppressive chemotherapy adjunct, myelosuppression | $1.2 Billion |
The costs of healthcare in Canada have risen considerably faster over the past few decades than the gross domestic product, and the expenditure on vision care has risen even faster, from 1.8% of total health expenditures in 1975 to 2.2% in 2007 [4]. The annual cost of vision loss in Canada due to age-related macular degeneration (AMD), diabetic retinopathy (DR), cataract, glaucoma, and refractive error was estimated at $15.80 billion (2007), with $8.6 billion resulting from direct healthcare expenditures, $4.4 billion from productivity loss (lower employment, higher absenteeism, and premature death), $1.8 billion from dead weight losses (welfare payments and lost taxation), $0.7 billion for care of people with vision loss, $305 million due to aids and home modifications, and $11.7 billion due to lost well-being. This equates to an annual financial cost of $19,370 per affected person, or $33,704 if the value of well-being is included [7]. The prevalence of vision loss is expected to increase from 2.5% of the population in 2007 to 4.0% in 2032.
The number of people over the age of 11 years with diabetes mellitus in England was estimated at 2,334,550 in 2010 with 166,325 (7.12%) believed to have DME. The total cost of health and social care attributed to these patients was £116,296,038 [28].
The cost to the German statutory health insurance system of caring for the ocular complications of diabetes was about є2.23 billion in 2002. German patients with DME use almost twice the medical resources as those with only mild retinopathy [15].
10.3 Cost-Effectiveness of Treating Diabetic Macular Edema
The concept of “cost-effectiveness” only has meaning when a particular intervention is compared to a standard [24]. Policy makers in the United States have generally not established formal standards for cost-effectiveness though they probably exist in an unpublished form [34]. Some other nations base standards of effectiveness on the quality-adjusted life year (QALY), a measure of health-related quality of life in which every health state is assigned a value between 1 (perfect health) and 0 (death) [12]. For ophthalmic conditions, most models are based on health states determined by visual acuities. A cost of $50,000/QALY is generally accepted as a willing-to-pay cost-effectiveness standard [17, 53], but some authors have suggested an upper limit of $100,000/QALY [25] (Fig. 10.1). The United Kingdom’s National Institute for Health and Care Excellence (NICE) generally approves interventions with cost-utility ratios of less than £20,000/QALY (approximately $32,000/QALY) but usually requires strong reasons to approve interventions costing more than $48,000/QALY. Some researchers have suggested that the United States should be willing to spend $100,000/QALY or more [52]. The World Health Organization recommends that interventions that cost less than the per capita gross domestic product per individual of a country per disability-adjusted life year are very cost-effective [51]. An intervention that costs less than 3 times the gross domestic product per disability-adjusted life year is considered cost-effective. According to these standards, ranibizumab remains cost-effective in the United States if 3.5 or fewer injections are required each year from years 3 through 14. Data from The Diabetic Retinopathy Clinical Research network Protocol I suggests that fewer injections are likely to be given after the first year of DME treatment, thereby suggesting that ranibizumab is cost-effective over extended periods of time [11]. Though cost-effective analyses are common in US medical literature, legislation in the United States has banned the use of QALY for making insurance coverage decisions [35].
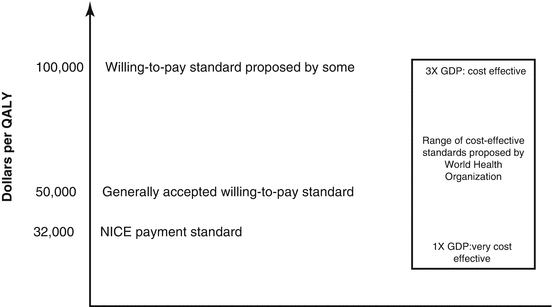

Fig. 10.1
The recommendations of cost-effectiveness standards made by several groups and government agencies. Some analysts have proposed that standards of over $100,000/QALY are still cost-effective. QALY quality adjusted life year, NICE United Kingdom’s National Institute for Health and Care Excellence, GDP gross domestic product
Laser photocoagulation and vitrectomy surgery are usually considered durable therapies for DR. Using data from the Early Treatment Diabetic Retinopathy Study [13], laser photocoagulation was estimated to produce a gain of 0.236 QALY and was considered to be highly cost-effective compared to no treatment [43]. Though repeat sessions of laser and return visits to the operating room are not uncommon for patients with advanced diabetic retinopathy, the total number of these interventions is generally far fewer than the number of required intravitreal anti-VEGF injections. Randomized, prospective trials suggest that patients require at least 15 anti-VEGF injections over the course of 3 years, and RISE and RIDE featured monthly injections for 36 months. In most vitreoretinal practices, physicians individualize therapy with pro re nata (PRN) or treat-and-extend (T&E) regimens. Compared to monthly injections, both strategies decrease the number of injections and T&E also decreases the number of clinic visits. Both regimens decrease the total direct costs for treating DME.
Treatment costs vary considerably depending upon the choice of drugs [42]. In the United States, compounded bevacizumab costs approximately $60 per dose, ranibizumab (0.3 mg) costs $1170 per dose, and aflibercept costs $1850 per dose [49, 50]. Triamcinolone (Kenalog®) costs only $49 per dose, the dexamethasone insert costs $1371 per dose, and the fluocinolone insert costs $8800 per dose. The total lifetime cost for one patient treated with anti-VEGF therapy has been estimated at $133,126. The cost to achieve one line of visual acuity improvement per year ranges from $60 to $561 [46]. When analyzed according to the incremental cost-effective ratio (ICER) per cost of QALY gained, each of the anti-VEGF drugs decreases costs compared to no treatment [18].
No trials have directly compared all of the available treatment options and regimens, and few have detailed the costs of therapy. The cost-effectiveness of therapy varies according to the authors – their assumptions and biases. The United Kingdom’s NICE evaluated an industry-sponsored study of ranibizumab for the treatment of DME and concluded that the cost-effectiveness of anti-VEGF therapy is not convincingly superior to laser photocoagulation [32]; two other studies, however, reached the opposite conclusion though they did not compare all treatment options or calculate lifetime costs [10, 29]. In the final step of NICE’s assessment of the cost-effectiveness of ranibizumab for DME, NICE used a preplanned subgroup threshold analysis to find that the ICER for ranibizumab treatment fell below the willing-to-pay threshold only for eyes with central retinal thickness greater than 400 μm at baseline. By approving ranibizumab use only for this subgroup, NICE effectively halved the potential payment budget for the treatment of DME [33].
Data from the RESTORE trial were used to create one of the first cost-effective analyses for the treatment of DME [29]. Using a Markov model, the authors determined that ranibizumab monotherapy resulted in a 0.17 QALY gain at an incremental cost of £4191 relative to laser monotherapy; this yields an ICER of £24,028. A probabilistic analysis reported a 64% probability that ranibizumab was cost-effective at a threshold of £30,000 per QALY. Combined ranibizumab and macular laser photocoagulation results in a 0.13 QALY gain over laser monotherapy at an incremental cost of £4695 with an ICER of £36,106. They concluded that ranibizumab monotherapy is cost-effective, but the cost-effectiveness of ranibizumab combined with laser photocoagulation is less certain because of higher costs and lower effectiveness. If a 40-year time line were used with an average patient age of 47 years – as in the study by Sharma – then the RESTORE model would predict a 0.26 QALY and an ICER of £10,412 for ranibizumab compared to laser.
A Markov model was used to determine the cost-effectiveness of different treatments (laser photocoagulation, intravitreal injections of triamcinolone acetonide, intravitreal injections of anti-VEGF drugs, or a combination of both) for DME [36]. The model determined that all treatments except laser monotherapy substantially reduced costs, and all treatments except triamcinolone increased quality-adjusted life years (QALYs). Combined laser photocoagulation and anti-VEGF therapy produced the greatest benefit by gaining 0.56 QALYs at a cost of $6975 for an incremental cost-effectiveness ratio per QALY compared to laser plus triamcinolone. Anti-VEGF monotherapy was similarly cost-effective to laser and anti-VEGF combination therapy. The authors concluded that anti-VEGF monotherapy or in combination with laser photocoagulation was the most effective treatment strategy for DME, and both compare favorably with cost-effective interventions for other conditions. According to the authors, the decision to add laser photocoagulation to anti-VEGF therapy should probably be made according to each patient’s preference. In most cases, adding laser to an anti-VEGF regimen does not incrementally improve VA beyond that achievable with anti-VEGF monotherapy. Laser minimally extends the durability of anti-VEGF therapy but introduces additional risk of permanent macular complications. Because of its low unit dose cost, the cost-effectiveness of bevacizumab exceeds that of ranibizumab. Absolute visual acuity gains with ranibizumab, however, are generally greater in eyes with moderate to severe vision loss, and this needs to be considered when choosing therapy.
A computer simulation cost-effectiveness analysis was based on 2-year data from DRCR.net Protocol I [10]. The authors calculated several ICER values in terms of dollars per letter: $393 (sham + laser vs. triamcinolone + laser); $5943 (ranibizumab + prompt laser vs. sham + laser); and $20 (ranibizumab + prompt laser vs. ranibizumab + deferred laser). In pseudophakic patients, the ICER value for ranibizumab + deferred laser (compared to triamcinolone + laser) was $14,690. In phakic patients, ranibizumab + deferred laser produced an additional 6 letters of visual acuity at a cost of $19,216 compared to triamcinolone + laser. The authors concluded that intravitreal triamcinolone acetonide appears to be the most cost-effective treatment for DME in pseudophakic eyes.
Using results from several of the earlier clinical trials, a cost-benefit analysis compared several treatments [46]. Not surprisingly, the use of lower cost drugs such as bevacizumab and triamcinolone proved to be most cost-effective [45].
A 14-year cost-utility analysis for the treatment of DME with ranibizumab was performed with data from the RISE and RIDE trials [2]. This analysis not only assessed the costs of ranibizumab therapy, but it contrasted these to savings gained from lowered vision-related healthcare costs. The 14-year gain attributed to ranibizumab therapy was 0.9981 QALYs, which produced an 11.6% improvement in quality of life. The direct ophthalmologic cost to treat one eye was $30,116 and for both eyes was $56,336. The decrease in direct nonophthalmologic medical costs from lower incidences of depression, injury, skilled nursing home admissions, and other costs associated with vision were $51,758. This resulted in a net medical cost of $4578. The mean cost to society for bilateral ranibizumab therapy was –$30,807, consisting of $31,406 in savings due to decreased caregiver costs and $3978 in decreased wage losses. The third-party insurer’s cost-utility ratio was $4587/QALY, and the societal cost perspective for bilateral therapy was –$30,807/QALY. This demonstrated that ranibizumab therapy not only produced a positive QALY of 0.9981 but also a positive financial gain of $30,807. The authors stated that ranibizumab produces a much greater gain than the 6–9% attributed to β-blocker treatment of systemic arterial hypertension, the 4–6% gain attributed to statin treatment of hyperlipidemia [1], and the 1% gain attributed to the use of bisphosphonates for osteoporosis [3].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree