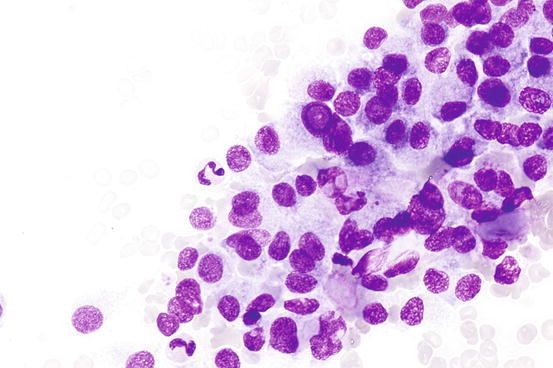
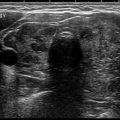
Fig. 7.1
Benign macrofollicular cluster with uniform, evenly spaced nuclei
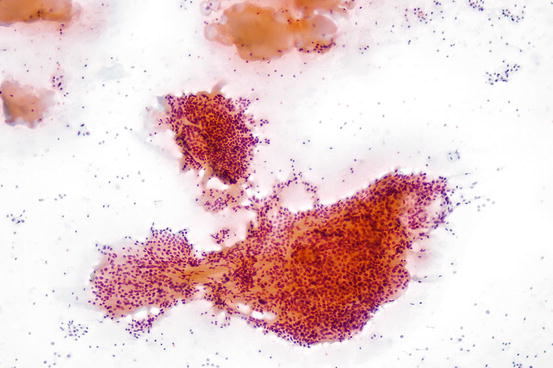
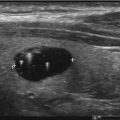
Fig. 7.2
Benign macrofollicular clusters and pools of colloid from a benign thyroid nodule aspirate
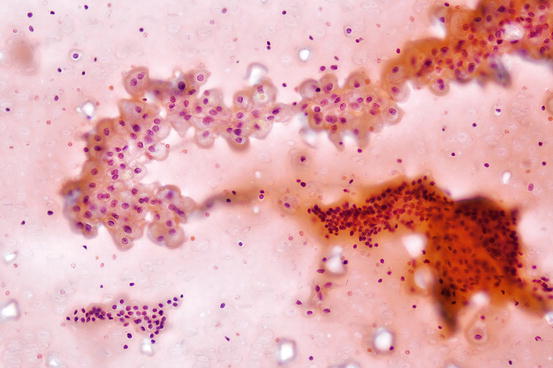
Fig. 7.3
Mixture of histiocytes and macrofollicular clusters in benign cystic thyroid nodule
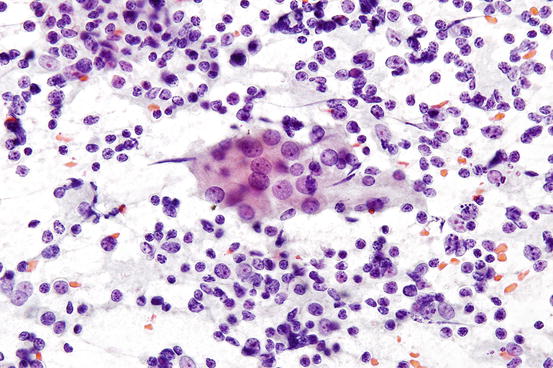
Fig. 7.4
Benign lymphocytes mixed with cluster of benign follicular cells in chronic lymphocytic thyroiditis
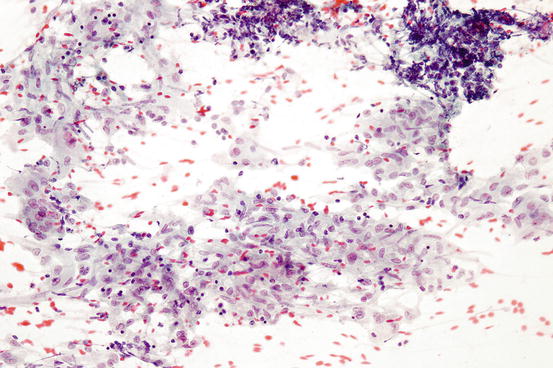
Fig. 7.5
Granulomatous inflammation characterized by clusters of epithelioid histiocytes
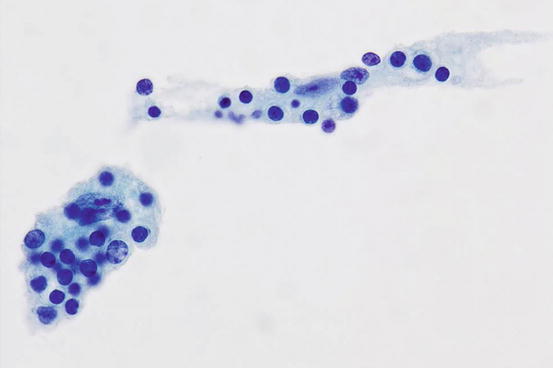
Fig. 7.6
Histiocytes in Rosai-Dorfman disease showing emperipolesis
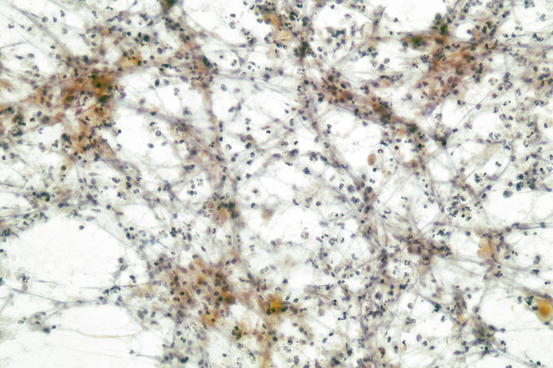
Fig. 7.7
Abundant neutrophils from an abscess of the thyroid
Papillary Thyroid Carcinoma
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid tumor representing approximately 80% of all thyroid malignancies. PTC is the most common pediatric thyroid malignancy and among adults occurs mostly in women. Histologically, papillary thyroid carcinomas may show papillary, solid, or follicular architecture. Some forms of papillary thyroid carcinoma may contain abundant granular and eosinophilic cytoplasm such as tall cell, oncocytic, and hobnail variants of PTC. Lamellar calcifications called psammoma bodies may be present and are more frequent in cases showing classical papillary architecture. While relatively diverse architectural patterns can be seen among papillary thyroid carcinomas, greater unity can be seen in its cytologic features. In particular, characteristic nuclear features are seen in most cases of papillary thyroid carcinoma.
Aspirates of papillary thyroid carcinoma usually produce cellular smears. Colloid is usually sparse or absent. The cellularity can even be appreciated before microscopic examination while the smears are being prepared. The smears of papillary thyroid carcinoma are frequently granular, producing a gritty sensation when the smears are being made. This contrast with FNAs is predominated by colloid in which the aspirated material spreads smoothly on the slides and shows a glistening quality. Microscopically, a well-sampled papillary thyroid carcinoma shows numerous clusters of follicular cells spread across the slide. Single cells can be seen, but numerous other diagnostic possibilities should be excluded such as medullary thyroid carcinoma. When colloid is present, it may have a dense “bubblegum”-like quality. Care should be taken to not confuse dense colloid with amyloid which would raise the possibility of amyloidosis or medullary thyroid carcinoma. Clusters in PTC may appear flat or rounded and may show a trabecular or branched appearance. Papillae may be seen with a characteristic central fibrovascular core (Fig. 7.8). In some cases the tips of the papillae may become detached having the appearance of caps. Small follicular groups can also be seen and may predominate simulating the appearance of a follicular neoplasm. Histiocytes and multinucleated giant cells may also be seen. Giant cells with a twisted appearance or scalloped edges are frequently associated with papillary thyroid carcinoma. In cases where follicular cells are infrequent or absent, the presence of such giant cells may merit a comment in the report to alert the clinician about the possibility of papillary thyroid carcinoma. Psammoma bodies, when identified, are a worrisome feature for papillary thyroid carcinoma. However, the presence of psammoma bodies alone is not specific for PTC and can also be seen in nodular hyperplasia, chronic lymphocytic thyroiditis, and Hurthle cell neoplasms [31, 32]. The tumor cells of PTC can show a variety of shapes including polygonal, cuboidal, or columnar contours. The cytoplasm varies from delicate and histiocytoid to dense and squamoid. Squamous metaplasia may be seen in papillary thyroid carcinoma, especially after prior biopsy or as a result of infarction. Squamous metaplasia may be accompanied by larger and more irregular nuclei than seen in typical PTC. This can sometimes create confusion with anaplastic thyroid carcinoma or metastatic squamous cell carcinoma from other sites. Knowledge of the clinical history including any prior biopsies performed in the thyroid may assist in raising the possibility of squamous metaplasia. In addition, while squamous metaplasia in PTC may have more cytologic atypia than usual PTC, the level of pleomorphism and nuclear atypia is usually far less than seen with anaplastic thyroid carcinoma or squamous cell carcinoma. The nuclei in squamous metaplasia may retain some similarities to usual PTC such as fine chromatin, grooves, or rare pseudoinclusions. It should be noted that squamous metaplasia can also involve benign thyroid follicles, especially in the setting of a cyst or after prior FNA.
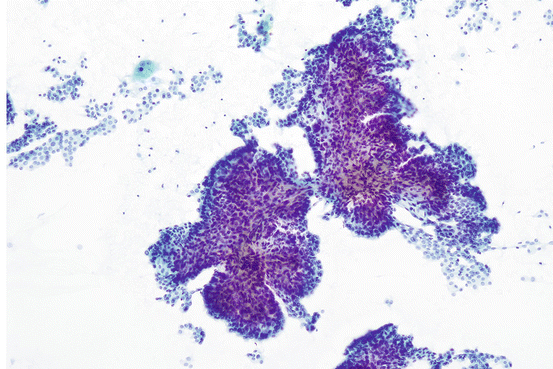

Fig. 7.8
Papillary clusters of papillary thyroid carcinoma
The nuclei in papillary thyroid carcinoma have shared features across most of the variants of PTC. On low power, the nuclei of PTC usually overlap, departing from the honeycombed appearance seen in benign thyroid aspirates. In some cases, the nuclei have an ovoid appearance, overlapping along the tips of the long axis of the nuclei (head-to-tail). This may create the appearance of nuclear streaming. In the most dramatic examples, the streaming nuclei form distinctive whorls (Fig. 7.9). The chromatin may appear fine and powdery or may have a ground-glass appearance. Nuclear membranes frequently show irregularities that manifest as nuclear grooves and pseudoinclusions (Fig. 7.10). In addition, rather than having a smooth appearance, the nuclear membranes may show clefts or sawtooth-like irregularities. The nuclear membranes usually appear thicker compared to the nuclei in benign follicular clusters due to margination of the chromatin. While it should be noted that grooves and pseudoinclusions are characteristics of PTC, they are not completely specific for PTC. Pseudoinclusions can sometimes be seen in other benign and malignant thyroid tumors and rarely can occur in nonneoplastic thyroid conditions [33]. Therefore, the diagnosis of PTC rests on a collection of observations and not on any one single criteria alone. Numerous pseudoinclusions can be seen in hyalinizing trabecular tumor. Aspirates of this benign tumor can have clusters showing enlarged overlapping nuclei with fine chromatin, grooves, and pseudoinclusions [34]. The number of pseudoinclusions usually far exceeds what is usually seen in PTC which can be a clue to the diagnosis. Other features include cells with fusiform contours containing oncocytic cytoplasm arranged in trabecular aggregates. Fragments of hyalinized stromal tissue (best seen on Diff-Quik), closely associated with the abovementioned clusters, are an additional useful clue.
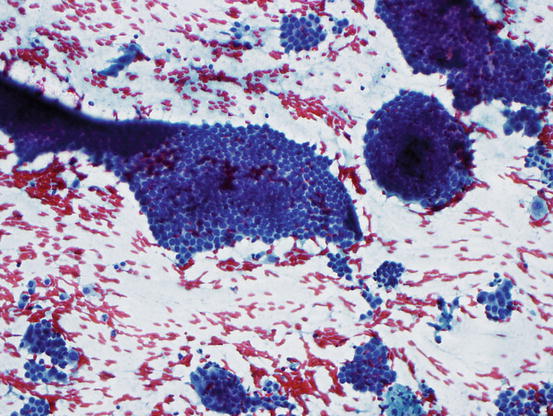
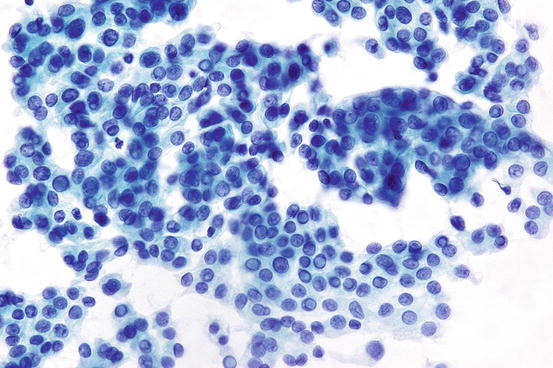

Fig. 7.9
Papillary thyroid carcinoma showing whorled clusters of follicular cells

Fig. 7.10
Clusters of follicular cells from papillary thyroid carcinoma showing several nuclear pseudoinclusions
Variants of papillary thyroid carcinoma have features that may be discernable by FNA. The cells of the tall cell variant of papillary thyroid carcinoma contain abundant granular cytoplasm and distinct cell borders. When seen on the edge, the abundant apical cytoplasm can be observed (Fig. 7.11). The tall cell variant of PTC may appear similar to Hurthle cell neoplasms. The presence of typical PTC nuclear features can help discriminate these two possibilities [35]. The columnar cell variant (CMV) of papillary thyroid carcinoma shows features that can resemble endometrioid or colorectal adenocarcinoma. Aspirates of CMV can show pseudostratified nuclei resembling a picket fence arrangement [36]. However, nuclear grooves are infrequent, and pseudoinclusions are usually absent. The diffuse sclerosing variant (DSV) of papillary thyroid carcinoma is observed mostly within the pediatric age range. Cytologically, in addition to the classic nuclear features seen with classical PTC, clusters of tumor cell showing squamous metaplasia are frequent in DSV. In addition, numerous psammoma bodies are observed. Many lymphocytes can be seen in the background of aspirates from DSV, which may cause diagnostic confusion with reactive changes associated with chronic lymphocytic thyroiditis. The follicular variant (FV) of papillary thyroid carcinoma can be difficult to diagnose on fine needle aspiration. For infiltrative types of FV, the cytological features are usually indistinguishable from classic PTC. However, the encapsulated follicular variant of PTC often shows less obvious nuclear features. Pseudoinclusions are usually absent, and nuclear grooves are less frequent than classical PTC. Aspiration specimens from the encapsulated follicular variant of PTC can mimic the appearance of follicular neoplasms or have atypical features that might be ascribed to the AUS/FLUS category. As mentioned earlier, a recent consensus has suggested that the noninvasive encapsulated FV be renamed the noninvasive follicular thyroid neoplasm with papillary-like features (NIFTP). Indeed, the encapsulated noninvasive follicular variant of PTC (now NIFTP) has more similarities to follicular neoplasms than PTC on both a molecular and clinical level. The new proposed nomenclature will most likely affect the risk of malignancy associated with class III and IV FNAs to some degree.
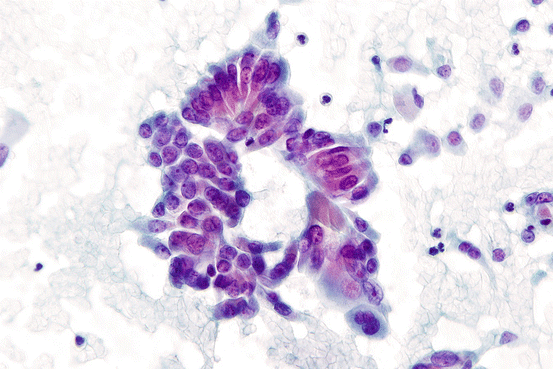

Fig. 7.11
Cluster seen on the edge from tall cell variant of papillary thyroid carcinoma
Follicular Neoplasms
Assessment of follicular nodules is one of the most diagnostically challenging areas in thyroid pathology. The histological distinction between a hyperplastic nodule and follicular neoplasm is based on whether the tumor has a capsule as well as distinct cytology and architecture from the surrounding. However, these features are somewhat arbitrary as all the abovementioned features have broad zones of overlap between follicular neoplasms and hyperplastic nodules. The distinction between follicular adenoma and follicular carcinoma is based solely on the presence or absence of invasive features. For the cytopathologist, this means there are no reliable features to distinguish between follicular adenoma and follicular carcinoma. The diagnosis of suspicious for follicular neoplasm or suggestive of follicular neoplasm is usually the greatest level of certainty that cytopathologist can render for this tumor type. Patients with this diagnosis may be referred to surgery in order to determine if the tumor is benign or malignant histologically.
Aspirates of follicular neoplasms can be hypercellular compared to hyperplastic thyroid nodules. However, the suspicion of follicular neoplasm should not rest on cellularity alone as cellular hyperplastic nodules can also produce hypercellular smears. If the increased cellularity is accompanied by the absence of colloid, this usually raises the concern for a neoplasm, and the possibility of a follicular neoplasm can be considered. In aspirates of follicular neoplasms, the follicular cells may be arranged in small ringlike clusters of 6–12 follicular cells called microfollicles (Fig. 7.12). When the smears are comprised of mostly microfollicles with absent or only spare colloid in the background, the risk of a follicular neoplasm is higher. Care should be taken to avoid overdiagnosing macrofollicles disrupted by shearing forces in smears as microfollicles. This artifact can be detected by observing the presence of the smaller clusters appearing to trail behind larger macrofollicular clusters. The disruption of macrofollicles into small microfollicle-like clusters is a relatively common artifact. If ample colloid is seen in the background, attention should be given to the possible presence of this artifact in order to avoid overcalling hyperplastic nodules as suspicious for follicular neoplasm. As some follicular neoplasms are hypervascular, abundant blood may herald the presence a follicular neoplasm. However, if the cellularity of the specimen is low, caution should be exercised as bloody aspirates may also signal poor aspiration technique or even the presence of a hematoma or vascular tumor.
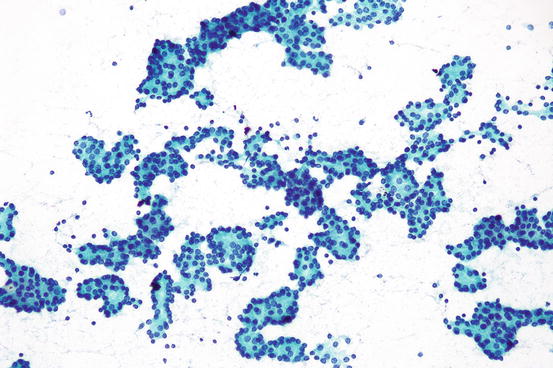

Fig. 7.12
Clusters of microfollicles , suspicious for a follicular neoplasm
Hurthle Cell Neoplasms
Hurthle (oncocytic) cell neoplasms are considered as a subtype of follicular neoplasms by the 2004 WHO. However, Hurtle cell carcinomas show distinct molecular abnormalities that may separate these tumors from follicular carcinomas [37]. In FNA specimens, Hurthle cell neoplasms are either exclusively or predominantly comprised of cells with abundant granular cytoplasm [38]. On Papanicolaou stains, the granularity imparts a deep blue color. The nuclei are enlarged and usually contain prominent central nucleoli. At times, the nuclei have relatively monomorphous shapes indicating a clonal appearance. However, significant nuclear size variation may be seen. Nuclear pleomorphism is not a sign of malignancy but rather can be seen in benign Hurthle cell neoplasms as well as nodular hyperplasia accompanied by Hurthle cell metaplasia. The nuclei may be eccentrically displaced imparting a plasmacytoid appearance (Fig. 7.13). Binucleated cells may be seen. The cells may be arranged as large clusters or microfollicles. Large clusters may have a three-dimensional appearance or appear as flat sheets. The cell clusters of Hurtle cell neoplasms show a tendency for less cohesion, with single cells present in the background. Moreover, some Hurtle cell neoplasm aspirates are dominated by single cells. When numerous single Hurthle cells are present, care should be taken to exclude the possibility of the oncocytic variant of medullary thyroid carcinoma, which can also show many single cells. In some cases the distinction can be subtle, and correlation with serum calcitonin levels may be required. Thin-walled blood vessels coursing through the tumor clusters, termed “transgressing vessels,” can be seen. When present, transgressing vessels are considered relatively specific for Hurthle cell neoplasms [39]. Several authors have attempted to determine the distinguishing features of benign and malignant Hurthle cell neoplasms. However, as with follicular neoplasms, no reliable cryptologic features separate Hurthle cell adenomas from Hurthle cell carcinoma.
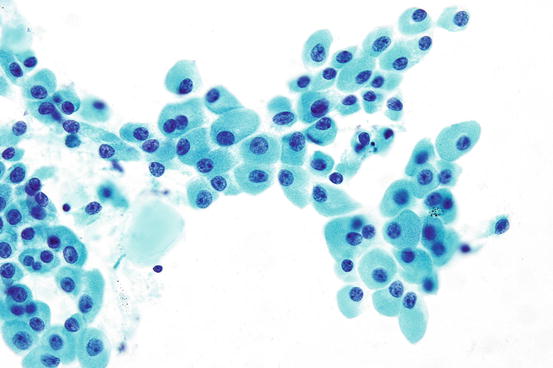

Fig. 7.13
Hurthle cells with abundant granular cytoplasm
Medullary Thyroid Carcinoma
Medullary thyroid carcinoma (MTC) represents approximately 10% of all thyroid malignancies. It arises from parafollicular C cells which maintain serum calcium homeostasis by the secretion of calcitonin. Medullary thyroid carcinoma can occur sporadically or in association with heritable genetic syndromes such as familial medullary thyroid carcinoma and multiple endocrine neoplasia types 2A and 2B. Fine needle aspirates of MTC can show a great degree of variability that can mimic other thyroid tumors. Smears usually show loosely cohesive aggregates and single cells. Single cells frequently predominate. The cells may have a variety of shapes including rounded, spindled, and triangular. Multinucleated tumor cells can also be present. All these shapes may be represented on a single aspirate. This variability can be an important clue to the diagnosis of MTC in conjunction with other findings [40]. Microfollicle-like clusters may be seen that can be mistaken for a follicular neoplasm. The cells contain moderately dense cytoplasm with granules that appear metachromatic on Diff-Quik stains. The nuclei are generally oval and may appear monomorphous; however, it is not uncommon to see at least scattered cells with markedly enlarged nuclei. This feature, called endocrine anaplasia, may cause confusion with higher-grade tumors such as anaplastic thyroid carcinoma. The nuclear chromatin of MTC appears granular which is commonly referred to as “salt-and-pepper chromatin.” Nuclear pseudoinclusions can be observed in MTC, mimicking the appearance of papillary thyroid carcinoma (Fig. 7.14). As many MTCs are dominated by single cells, or loosely cohesive aggregates, the low power pattern of the smear can assist in avoiding this pitfall. Up to 80–85% of medullary thyroid carcinomas are associated with amyloid production composed of calcitonin or calcitonin-related peptide in most cases [41]. In aspirates this is seen as amorphous globular deposits that may be intimately associated with the tumor cells (Fig. 7.15). In most cases it is useful to have additional material for a cellblock preparation in order to perform immunohistochemical testing for calcitonin.