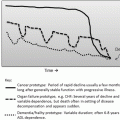
Fig. 2.1
Multifactorial model of delirium . The risk of a delirium is a combination of extrinsic factors to the patient (e.g., severity of medical illness, stress of surgical intervention), intrinsic factors to the patient (e.g., cognitive impairment, advanced age), and iatrogenic factors (e.g., sleep disruption, pain control)
Routine hospital care introduces external iatrogenic risk factors, including polypharmacy (discussed fully in a separate chapter), disruption of sleep–wake cycles, infection, psychoactive medication prescription (specifically benzodiazepines and anti-cholinergic drugs), physical restraints, use of bladder catheters, and iatrogenic adverse events have all been identified as risk factors for delirium [14]. See Table 2.1 for a summary of delirium risk factors.
Table 2.1
Risk factors for delirium
Advancing age |
Impaired cognition (e.g., dementia) |
Severe illness or comorbidity burden |
Functional dependence |
Infection or sepsis |
Hearing or vision impairment |
Sleep disturbance |
Depression |
Poor nutrition |
Anemia |
Alcohol use |
Hypoxia or hypercarbia |
Dehydration |
Electrolyte abnormalities |
Inappropriate medication prescription • >5 new medications • benzodiazepines • anticholinergics • antihistamines • antipsychotics |
Various specialty-specific rates of delirium have been reported that further identify groups of hospitalized patients who are more at risk for the development of delirium. Patients who present to the emergency department or are in the intensive care unit, oncology patients, and patients for multiple surgical specialties (e.g., vascular or orthopedic surgery) can have higher rates of delirium than the average hospitalized adult. Ten percent of patients present to the emergency department with delirium, although this number may under-represent the true incidence [13, 15]. Orthopedic injuries and operations also carry high risk, with 40 % of patients developing delirium after bilateral knee replacement [16] and up to 60 % following hip fracture [17]. Patients undergoing coronary artery bypass grafting have rates of postoperative delirium of 33–50 % [18, 19].
Intensive care unit (ICU) patients , both medical and surgical, are at extremely high risk of delirium. The prevalence of delirium has been reported to be as high as 80 % [20]. There is, however, dramatic variability in the incidence of delirium in the ICU. Recently, because of the recognition of the risk of delirium, many ICUs have specific pathways for delirium prevention, which can significantly reduce the occurrence of delirium [21, 22]. ICU care is associated with disruption of sleep–wake cycling, high severity of illness, and use of many drugs that are associated with increased risk of delirium, so it is unsurprising these patients are more vulnerable to developing delirium.
2.4 Presentation of Delirium
Delirium is exceptionally heterogeneous in its presentation. The fact that the course of delirium waxes and wanes makes the diagnosis of delirium clinically challenging. This has led to a wide variety of diagnostic tools which can be used to diagnose delirium (see “Diagnostic Tools” section below and Chap. 8, Screening Tools for Geriatric Assessment by Specialists).
While there are several ways to define subtypes of delirium, one of the most commonly used strata is by motor activity, known as hyperactive, hypoactive, and mixed subtypes of delirium (see Fig. 2.2) [23]. The primary distinction between these motor subtypes is the presence of agitation versus lethargy in the patient’s clinical presentation. Patients with evidence of both hyperactive and hypoactive delirium are described as having mixed delirium.
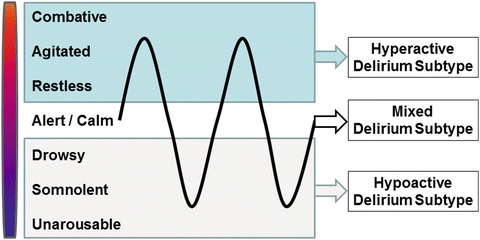

Fig. 2.2
The motor subtypes of delirium. The motor subtypes of delirium include hyperactive (pure overactive state represented in blue), hypoactive (pure underactive state represented in gray), and mixed (fluctuation between over- and underactive represented by black line)
There are several checklists (see section below) that identify psychomotor symptoms that are associated with delirium, and when present in combination, increase the specificity of these symptoms to delirium [24]. Hyperactivity in delirium may be associated with increased involuntary movements, restlessness, wandering, increased speed, amount, or volume of speech, inability to sleep, distractibility, combativeness, hallucinations, or tangential thoughts (among others). Hypoactive delirium may present as apathy, decreased activity, decreased speed, amount, or volume of speech, somnolence, or decreased alertness. A mixed subtype presentation occurs when patient symptoms fluctuate between these two categories of agitation and lethargy.
Hypoactive delirium may be under-represented in the epidemiology of delirium because it is difficult to diagnose [25, 26]. A high level of clinical vigilance and suspicion of the diagnosis of delirium is especially necessary to diagnose hypoactive delirium. Hypoactive symptoms may be easy to attribute to other patient health conditions without a high clinical suspicion to monitor for delirium. Further, some studies have demonstrated that postoperative patients with hypoactive delirium have worse prognosis when monitoring 6-month mortality rate [27], although other studies have demonstrated improved outcomes for patients with hypoactive delirium [28].
2.5 Diagnostic Tools for Delirium
There are many diagnostic tools to identify delirium. They can be specifically designed for the ICU patient or other clinical settings, and may focus on certain diagnostic criteria, such as motor subtype. Below are brief descriptions of some commonly used diagnostic tools and comments about specific indications or limitations.
The confusion assessment method (CAM) is the most widely recognized tool to assess delirium and can be completed in under 5 min. [29] It uses four criteria: (1) acute onset of symptoms with fluctuating course, (2) inattention, (3) disorganized thinking, and (4) altered level of consciousness. The first 2 criteria must be present with either the 3rd or the 4th criteria. It has high inter-rater reliability with high accuracy compared to psychiatrist assessment for delirium.
The Delirium Rating Scale-Revised-98 (DRS-R98) is a 16-item scale, of which 13 items score for severity of symptoms. It has high inter-rater reliability, sensitivity, and specificity, including use in patients who have concomitant neurologic disease, such as dementia [30]. It is designed for use by any healthcare professional.
The cognitive test for delirium (CTD) is a diagnostic test specifically designed to assess critically ill hospitalized patients, including patients unable to communicate, such as those who are intubated and sedated [31]. It particularly emphasizes nonverbal domains, specifically visual and auditory symptoms. It is also able to reliably distinguish the difference between delirium and other psychiatric disorders.
The Delirium Motor Subtype Scale (DMSS) is used specifically to identify features of hyperactive and hypoactive delirium [24]. It is an 11-point scale any healthcare provider can use to assess patient behaviors, and includes 7 hypoactive features and 4 hyperactive features. Two symptoms must be present in order to classify delirium in a specific subtype.
The CAM for the Intensive Care Unit (CAM-ICU) was developed from the CAM assessment to better diagnose patients who are mechanically ventilated [32]. It uses nonverbal assessments to identify the same criteria of acute onset of symptoms with fluctuating course, inattention, and disorganized thinking or altered level of consciousness. It has high levels of sensitivity and specificity for delirium in ventilated patients, although the traditional CAM is more effective in patients able to fully participate in the assessment [20].
The intensive care delirium screening checklist is another test for patients in the ICU setting. It is a brief checklist of eight items based off of DSM criteria of delirium [33]. While it also has high sensitivity for delirium in the ICU, it is less specific than the CAM-ICU method. It is designed for use for all healthcare professionals.
The Memorial Delirium Assessment Scale was specifically developed to monitor development of delirium in ill patients enrolled in clinical trials [34]. It involves a 10-item checklist which was validated in patients with AIDS and metastatic cancer. It is well suited for use in repeated assessments over time for patients being seen longitudinally in trials.
The important issue is that a clinician should be very familiar with one or two of these screening tools and use them in daily practice.
2.6 Medical Evaluation of Delirium
Given the heterogeneous presentation of the clinical syndrome of delirium in combination with the complex intrinsic and iatrogenic precipitating factors, a structured, thorough, and routine approach to evaluation of the patient with delirium is necessary. A hospitalized patient may have presented at admission with delirium or develop it during their hospital course. While it is not only important to recognize the clinical syndrome, it is also important to identify correctable conditions which contributed to the state of delirium. Acute onset of delirium may have developed secondary to a single provocative factor (such as a symptomatic urinary tract infection (UTI) , myocardial infarction (MI)), multiple medications (polypharmacy), admission to ICU, and others).
The appropriate workup of delirium involves methodical evaluation of the patient to identify treatable causes as well as initiate behavioral interventions. Table 2.2 outlines a comprehensive workup for patients with acute delirium which should supplement bedside examination. While many of these tests should be considered to be routine in an acute clinical change, others should only be considered if clinically indicated.
Table 2.2
Medical evaluation of delirium
Routinely ordered | Ordered if indicated | |
|---|---|---|
Laboratory tests | Complete blood count (infection, anemia) Basic metabolic panel (electrolyte disturbances, acid base status, renal function) Glucose (hypo- or hyper-glycemia) Arterial blood gas (hypoxia or hypercarbia) Urine analysis (infection but asymptomatic bacteriuria is not thought to cause delirium and is very common in older patients, especially women | Troponin (myocardial infarction) Thyroid levels (hypo- or hyper-thyroidism) ESR (inflammation) Viral titers or bacterial cultures (infection) Urine or blood drug screen (intoxication) Thiamine and Vitamin B12 (vitamin deficiency) HIV (infection) Sputum culture Blood culture |
Imaging | Chest X-ray (infection) | Head CT (dementia, stroke) Brain MRI (dementia, stroke) |
Clinical evaluation | Physical examination Medication review (BEERs list) [52] Social history (alcohol or benzo use) | Remove un-needed catheters |
Ancillary tests | EKG (myocardial infarction) Pulse oximetry (hypoxia) | EEG (seizures, metabolic disturbance) Lumbar puncture (meningitis) |
2.7 Prevention of Delirium
Although recognition and treatment of delirium once the patient develops the syndrome is essential, interventions to prevent delirium occurrence are essential for all patients at risk for delirium. Identification of individuals with multiple risk factors (e.g., frail, elderly, multiple comorbidities) allows the clinician to target preventive interventions to the at-risk population. Interventions such as making sure the patient has full use of their sensory aids, orientation protocols, early mobilization measures, minimization of sleep disturbance, and avoidance or discontinuation of high risk medications can all create an environment that will lower the risk of delirium for the at-risk patient [35]. Daily rounds that address these non-pharmacologic interventions utilize a multidisciplinary care team and plan that creates consistent assessment of these issues. Up to 40 % of hospitalized patients may have preventable delirium [14, 28]. Both of the current clinical practice guideline statements strongly recommend the implementation of multi-component delirium prevention protocols for patients at risk for delirium [35, 36],
Educational programs concerning delirium in every medical center are essential. These programs should be considered a system-level prevention tool. Education of healthcare providers about recognition, prevention, and treatment of delirium consistently reduces episodes of and duration of delirium, regardless of the specific intervention or protocol. [37–39] Further, educational interventions are cost-effective and associated with no patient harm [40–42].
2.8 Treatment of Delirium
When a patient does develop acute delirium, management of a potential underlying reversible cause of the delirium is essential. Appropriate treatment of identifiable causes will improve the patient’s clinical condition. However, risks and benefits of aggressive or interventional therapies should be considered when treating a delirious patient, and weighed in the context of their clinical condition and goals of care. See Table 2.3 for modifiable causes of delirium with a proposed intervention. Behavioral modifications have been described above in the section regarding prevention of delirium. Interventions such as encouraging use of sensory aids, establishing day–night cycling, and the other interventions described in the previous section are effective in treating delirium in addition to their role in prevention.
Table 2.3




Factors that cause delirium which can be clinically addressed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree