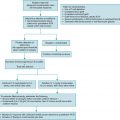
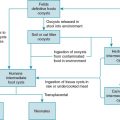
Viral infections represent a significant cause of morbidity and mortality among children who have undergone either solid organ (SOT) or hematopoietic stem cell transplantation (HSCT), as well as in children undergoing therapy for malignancies. Herpesviruses, which possess a unique ability in their life cycle to establish latent infection, are especially significant within these patient populations because of their capacity to reactivate in the setting of immunosuppression. Cytomegalovirus (CMV) is the most common herpesvirus causing disease among adult transplant recipients and represents an especially important pathogen among pediatric immunocompromised patients.
CMV is a β-herpesvirus that shares many of the characteristics of other herpesviruses. It has a large, double-stranded DNA genome with a viral capsid and envelope, and core of proteins that are conserved within all of the herpesviruses. The pathogenesis of CMV in otherwise healthy individuals is directed by viral transcripts that interfere with viral antigen presentation on the cell surface and lead to lytic viral replication. Symptoms that occur with viral replication are primarily associated with the immune response to the infection rather than direct viral destruction; therefore symptoms may be blunted in immunocompromised hosts with an ineffective immune response. Most healthy hosts are asymptomatic with CMV infection but shed virus in saliva and other mucous membranes, leading to transmission between hosts.
CMV infects epithelial cells, lymphocytes, and monocytes and ultimately establishes latency within these cells as well as in macrophages through a balance of viral immune evasion mechanisms and host immune control. The innate immune response through Toll-like receptor (TLR) inflammatory pathways, as well as memory natural killer (NK) cells and γδ T cells, is important for initial control of CMV replication and disease. This primary immune response is sufficiently restricted, however, to allow the virus to establish latency rather than be eradicated. , Adaptive immunity mediated through CD4 + and CD8 + T-cell responses is necessary for immune surveillance to prevent viral reactivation and proliferation of virus-infected cells. Humoral immune responses mediated by B cells are also needed for immune memory, but viral reactivation can occur despite adequate antibody responses in infected individuals. In transplant recipients, the disruption of effector T cells through immunosuppressive therapies leads to the potential for viral reactivation, replication, and associated CMV disease. Similarly, in oncology patients, chemotherapeutic agents as well as lymphoid malignancies that impair T-cell function promote CMV replication and may lead to disease. An unusual feature of herpesviruses, especially CMV, is the ability to precipitate graft rejection, graft-versus-host-disease (GVHD), autoimmunity, malignancies, and lead to a heightened risk of other opportunistic infections. This concept is known as heterologous immunity and is characterized by alteration of the immune response to new pathogens or to the transplanted graft by memory immune responses to the previously encountered viral pathogen. , These varied interactions of CMV with the immune system and the balance between disease owing to viral replication versus graft rejection present challenges for effective management of infection in immunosuppressed transplant recipients.
Epidemiology and risk factors
Seroprevalence rates of CMV in the general population have been reported to range from 30% to 97%; therefore it is likely that many children will develop CMV infection during their lifetime. CMV is ubiquitous, and transmission occurs horizontally through person-to-person contact with virus-containing secretions from infected individuals (saliva, urine, genital secretions), vertically from mother to infant (in utero, during delivery, or postnatally through breastfeeding), through transfusions of blood products from infected donors, and through SOT or HSCT from infected donors. Among children in day care settings, transmission through urine and saliva is frequent and represents a common scenario for primary CMV acquisition. Transmission among household contacts also occurs frequently. Children with primary CMV acquisition often shed virus for prolonged periods, leading to the potential for ongoing transmission to other children and caregivers. Sexual transmission among adolescents and adults also occurs.
In the setting of SOT, individuals who are CMV seronegative before transplantation and receive an organ from a seropositive donor ([D]-positive/recipient [R]-negative: D + /R − ) are at high risk of primary CMV infection at the time of organ transplantation and ultimate development of CMV disease. Children are more likely to be CMV seronegative at the time of transplantation than adults and are at risk for newly acquiring CMV from their organ donor, particularly if the donor is an older child or adult. Before the routine use of antiviral prophylaxis, the incidence of symptomatic CMV infection among liver transplant recipients had been reported to be 20% to 60% within the first 30 to 90 days after SOT. Simultaneous receipt of induction immunosuppression may potentiate the risk of CMV disease among SOT recipients with donor and recipient seropositivity mismatch. SOT recipients who are previously CMV infected before transplantation (R + ) are at risk of CMV reactivation after initiation of immunosuppression after SOT. The risk of CMV disease among SOT recipients also varies depending on the transplanted organ, owing to variation in immunosuppressive strategies to prevent organ rejection. Lung and small intestine transplant recipients have a higher risk of developing CMV disease, even in the setting of recipient seropositivity before transplant, whereas liver, heart, and kidney recipients have a lower risk of reactivation. Risk assessment of SOT recipients based on donor and recipient serostatus is summarized in Table 17.1 .
| Organ | Donor CMV Serostatus | Recipient CMV Serostatus | Risk Assessment |
|---|---|---|---|
| Kidney, liver, heart | Negative | Negative | Low |
| Positive or negative | Positive | Intermediate | |
| Positive | Negative | High | |
| Lung, intestine | Negative | Negative | Low |
| Positive or negative | Positive | High | |
| Positive | Negative | High |
Recipients of allogeneic HSCT who are CMV seropositive but receive cells from a CMV- seronegative donor or from cord blood (D − /R + ) are similarly at risk of primary CMV infection and heightened potential for CMV disease. Risk assessment of HSCT recipients is summarized in Table 17.2 . T-cell lymphopenia and prophylaxis against GVHD also contribute to the risk for CMV disease. CMV-infected HSCT recipients who undergo unrelated donor or mismatched donor transplants have higher rates of CMV reactivation or disease compared with those who undergo matched, related transplantation. HSCT recipients who require increased immunosuppression for treatment of GVHD also have higher rates of CMV reactivation and disease, although autologous HSCT recipients generally have low rates of CMV reactivation and disease. In other immunocompromised hosts, such as patients with hematologic malignancies receiving chemotherapy, primary CMV acquisition through person-to-person contact or infected blood products or reactivation of previous CMV infection can create a risk for CMV disease given the effects of both the tumor and treatment on the immune system. Children with primary immunodeficiencies in whom HSCT is pursued as a curative treatment may have had CMV infection and disease before HSCT. In these patients, if T-cell immune dysfunction is significant, control of CMV before HSCT may be difficult and leads to greater risk of CMV reactivation after HSCT. Lymphoid malignancies are associated with a greater risk of CMV reactivation compared with myeloid malignancies, and certain therapies such as alemtuzumab, fludarabine, and rituximab are also associated with higher risk.
| Recipient CMV Serostatus | DONOR CMV SEROSTATUS | |
|---|---|---|
| Negative | Positive | |
| Negative | Low risk | Intermediate risk |
| Positive | High risk | Intermediate risk |
Clinical manifestations
Primary CMV infection in immunocompetent individuals is often asymptomatic but may manifest as a self-limited febrile illness or mononucleosis-like syndrome before establishing life-long latency. Among immunocompromised hosts, clinical manifestations may vary widely from asymptomatic replication to CMV-associated end-organ disease. Updated definitions to describe the various categories of CMV infection and disease have recently been published to standardize reporting of CMV-associated outcomes among transplant recipients, taking into consideration advanced diagnostic testing techniques. Additional definitions are also used to describe CMV characteristics in patients with hematologic malignancies or those undergoing HSCT. The terminology used throughout this chapter aligns with definitions from the American Society of Transplantation as well as the CMV Drug Development Forum recommendations for definitions used in clinical trials :
- •
Latent CMV: CMV seropositivity without infection/replication.
- •
CMV infection: Virus isolation or detection of viral antigens or nucleic acid in any body fluid sample or tissue. CMV replication is evidence of viral multiplication and may be used instead of CMV infection.
- •
Primary CMV infection: First detection of CMV infection in an individual with no evidence of prior CMV exposure before transplantation or other immunosuppression.
- •
Recurrent CMV infection: New CMV infection in an individual with previous evidence of CMV infection in whom the virus has not been detected for at least 4 weeks during active surveillance. Recurrent infection may result from reactivation of latent virus (endogenous) or reinfection (exogenous).
- •
CMV syndrome: Detection of CMV in blood in the setting of a constellation of symptoms that may include fever, fatigue, leukopenia or neutropenia, or elevation of liver enzyme levels in an immunocompromised host.
- •
CMV disease: The combination of CMV detection or CMV syndrome plus end-organ disease. CMV disease often involves the transplanted organ in an SOT recipient but may affect many other organ systems as well. Table 17.3 summarizes the clinical manifestations and criteria required for proven CMV disease at various sites.
TABLE 17.3
Definitions of Cytomegalovirus Disease
From Ljungman P, Boeckh M, Hirsch H, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87-91.
Site of Disease
Clinical and Diagnostic Criteria
CMV syndrome
CMV detection a in blood plus two or more of the following symptoms: fever, malaise, leukopenia, neutropenia, thrombocytopenia, elevation of liver enzymes, elevation of % atypical lymphocytes.
CMV pneumonia
CMV detection in lung tissue or in bronchoalveolar lavage fluid plus clinical symptoms and/or signs of pneumonia such as new infiltrates on imaging, tachypnea, hypoxia. CMV detection by bronchoalveolar lavage suggests probable disease, not proven disease.
CMV gastrointestinal disease (e.g., colitis, esophagitis)
CMV detection in biopsy tissue with macroscopic mucosal lesions and upper and/or lower gastrointestinal tract symptoms and/or signs. In HSCT recipients, information regarding the presence of absence of graft-versus-host-disease on histopathology should also be included.
CMV hepatitis
CMV detection in liver biopsy tissue plus presence of abnormal liver enzymes.
CMV ventriculitis/encephalitis
CMV detection in tissue plus clinical symptoms and/or signs of central nervous system infection. CMV detection in cerebrospinal fluid suggests probable disease, not proven.
CMV nephritis
CMV detection in renal allograft tissue in the setting of renal dysfunction and histologic features of CMV infection. Detection of CMV in urine is not sufficient for diagnosis, as asymptomatic viral shedding in urine is common.
CMV cystitis
CMV detection in bladder biopsy in a patient with clinical symptoms and/or signs of cystitis. Detection of CMV in urine is not sufficient for diagnosis, as asymptomatic viral shedding in urine is common.
CMV myocarditis
CMV detection in heart biopsy specimen in a patient with clinical symptoms and/or signs of myocarditis.
CMV retinitis
Typical ophthalmologic signs identified by an ophthalmologist experienced with CMV retinitis. Although CMV detection in vitreous fluid is recommended, it is not required for this diagnosis.
Other organ involvement
CMV detection in tissue of ot her organs and compatible clinical symptoms and/or signs of specific organ involvement.
CMV, cytomegalovirus.
a CMV detection methods in biopsy or other samples include virus isolation, rapid culture, immunohistochemical analysis, in situ hybridization, nucleic acid testing, and quantitative polymerase chain assay.
Primary CMV infection from a graft, CMV reactivation, and CMV disease were traditionally most likely to occur within the first 3 months after OT or within the first 100 days after HSCT. , CMV reactivation in HSCT recipients during this period could also manifest as delayed engraftment. Implementation of prophylaxis strategies with antiviral agents during this early stage after SOT or after HSCT has shifted the timeline for reactivation and disease to periods after prophylaxis has been discontinued. Immunocompromised children who acquire primary CMV infection through community exposures are at risk for prolonged CMV replication and disease, particularly SOT recipients who remain to take lifelong immunosuppression, compared with immunocompetent children with community acquisition. Primary CMV infection or reactivation in the setting of immune dysfunction as the result of immunosuppression may also precipitate indirect effects of CMV infection, including organ rejection, GVHD, and disease due to opportunistic pathogens.
Disease prophylaxis and prevention
Table 17.4 summarizes the antiviral agents that may be used for CMV prophylaxis and treatment. Identification and prevention of CMV disease in immunocompromised pediatric patients, including HSCT, SOT, and those receiving cancer chemotherapy, is paramount to decreasing morbidity and mortality in these populations. Methods to prevent CMV disease can be classified as prophylaxis or preemptive. Prophylaxis occurs when antiviral therapy is provided to all (universal) or at-risk (targeted) patients for a predetermined period of time after transplantation. Preemptive therapy is the initiation of antiviral therapy with the identification of CMV replication, usually by quantitative polymerase chain reaction (PCR) during scheduled screenings at predetermined intervals, with or without evidence of symptoms of CMV. A mixed model of short-term prophylaxis followed by surveillance at scheduled intervals, currently referred to as “surveillance after prophylaxis”, can also be used. The advantages and risks of each prevention method must be assessed, including the risk for side effects, costs, and potential emergence of resistance with prophylaxis and the risk of indirect CMV effects, missed surveillance blood samplings, or delay in intervention with the preemptive approach. In general, prophylaxis is more commonly used in SOT and preemptive therapy is more common in HSCT.
| Agent | Route of Administration | Pediatric Dosing: Prevention | Pediatric Dosing: Treatment | Toxicity Monitoring |
|---|---|---|---|---|
| Ganciclovir | IV | 5 mg/kg q24h a (after 5-7 days of twice daily induction in HSCT) | 5 mg/kg q12h a | Leukopenia, renal dysfunction |
| Valganciclovir | Oral | 7xBSAxCrCl q24h a | 7xBSAxCrCl q12h a | Leukopenia, renal dysfunction; should be used with caution in patients with malabsorption such as recent small intestine transplant recipients |
| Foscarnet | IV | 60 mg/kg per dose q12h for 7 days, followed by 90-120 mg/kg per dose once daily until day 100 after HSCT a | 60 mg/kg/dose q12h for 7-14 days, followed by 90-120 mg/kg per dose once daily until CMV indicator is negative a | Renal dysfunction, electrolyte disturbances |
| Cidofovir | IV | Variable: 3-5 mg/kg weekly a ± probenicid | Variable: 3-5 mg/kg weekly a ± probenicid | Renal dysfunction |
| Letermovir | Oral | Only approved in adults; pediatric studies in development | Not approved for treatment | Interaction with tacrolimus, limited activity against other herpesviruses |
| CMV immune globulin | IV | Variable: 100-150 mg/kg per dose | Variable: 100-150 mg/kg per dose |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree