(1)
Mayo Clinic, Jacksonville, Florida, USA
6.1 Introduction
The use of the intraocular pharmaceutical agents that were discussed in Chapters 4 and 5 has transformed the treatment of diabetic retinopathy (DR). Before the introduction of potent pharmacotherapy for DR, early diagnosis of DR followed by prompt laser photocoagulation to eyes at risk of vision loss was the most effective way to stabilize vision [23]. Significant improvements in visual acuity (VA) were rarely achieved in patients with established diabetic macular edema (DME), so the primary goal of laser photocoagulation was to prevent further loss. Pars plana vitrectomy for persistent vitreous hemorrhage in eyes with healthy maculae sometimes produced dramatic improvements in VA, but, unfortunately, these were a small minority of patients with diabetes-related vision loss [52].
Administration of intravitreal pharmacotherapy (Fig. 6.1) now gives patients with vision loss due to DR an excellent chance of achieving a clinically meaningful (>5 ETDRS letters) improvement in VA. Effective treatment regimens usually require years of frequent assessments and several administrations of one or more drugs, often in conjunction with laser photocoagulation and surgery [8, 23]. These demanding regimens can be expensive and they challenge even the most compliant patients, but for patients and physicians who adhere to a sensible treatment strategy, the results can be gratifying and life-changing.
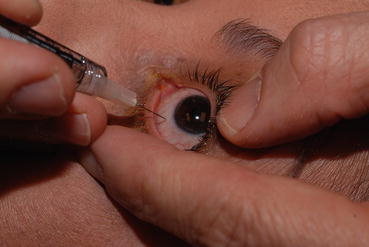

Fig. 6.1
This photograph demonstrates the technique used by the author for an intravitreal injection of a vascular endothelial growth factor inhibitory drug. An eyelid speculum is not used as the surgeon manually retracts the eyelids. Injecting the drug into the vitreous offers the following advantages over systemic administration: 1. Drug is deposited close to the target tissue. 2. The vitreous acts as a slow-release depot that naturally enables extended duration therapy. 3. Systemic exposure to the drug is limited because of the low total dose
The goal of this chapter is to synthesize the results produced by the randomized trials that were presented in previous chapters to propose data-driven – whenever possible – treatment guidelines that can be used in most clinical situations. Some new data will be introduced in this chapter, but most will be summarized from previously referenced trials. Despite the plethora of published manuscripts from the past 10 years that have provided level I and II evidence supporting the treatment of DR, conflicting results challenge certain recommendations, and significant gaps in our knowledge remain. Fortunately, however, our present knowledge enables us to make sound, evidence-based, scientific decisions when treating most patients.
6.2 General Medical Care
The first two chapters of this volume detailed the pathophysiology of DR, its important risk factors, and exacerbating conditions. To help patients with their ophthalmic and systemic health, ophthalmologists must remember that DR represents the ocular manifestation of a multisystem disease that includes both microvascular (nephropathy and neuropathy) and macrovascular (cardiovascular and cerebrovascular) conditions. Ophthalmologists have the opportunity to not only maintain and improve visual function but, through counseling of patients and communication with primary care physicians, to improve glucose control, minimize the progression of systemic vascular diseases, reduce morbidity, and prolong life expectancy. Ophthalmologists should develop and maintain close relationships with primary care physicians, internists, and endocrinologists. Important systemic interventions that may prevent or control DR are listed in Table 6.1.
Table 6.1
Systemic medical conditions that affect the development and progression of diabetic retinopathy
Systemic medical conditions and diabetic retinopathy | |
|---|---|
Study | Key findings |
Diabetes mellitus | |
UK Prospective Diabetes Study (UKPDS) | Type 1 diabetes mellitus 4209 patients (1977–1999) Intensive versus conventional therapy: 35% reduced progression per A1C point 47% reduction in moderate vision loss |
Diabetes Control and Complications Trial (DCCT) | Type 1 diabetes 1441 patients (1983–1993) Intensive versus conventional therapy (HbA1C 7.0 vs. 9.0) 50% reduction in advanced retinopathy Intensive control can temporarily worsen retinopathy in the short term |
Systemic arterial hypertension | |
Wisconsin Epidemiological Study of Diabetic Retinopathy | Progression of retinopathy was associated with: Higher diastolic blood pressure at baseline Increase in diastolic blood pressure over a 4-year period |
UK Prospective Diabetes Study (UKPDS) | Systolic blood pressure <150 mmHg: Decreased the progression of DR Decreased the need for macular laser photocoagulation for DME |
EUCLID study | Lisinopril decreased the progression of DR in normotensive type 1 diabetics |
DIabetic REtinopathy Candesartan Trials (DIRECT) | In type 1 diabetics, 5 years of treatment: Decreased the incidence of DR Had no effect on progression of established DR In type 2 diabetics: 34% regression of DR (P = 0.009) Less severe retinopathy in types 1 and 2 (P = 0.03) |
RASS trial | Evaluated 285 normotensive patients treated with enalapril, losartan, or placebo for 5 years Progression of retinopathy by 2 steps: Placebo (38%) Enalapril (25%; P = 0.02) Losartan (21%; P = 0.008) Enalapril and losartan increased the likelihood of less DR progression by 65 and 70% independent of blood pressure lowering |
Hyperlipidemia | |
Fenofibrate Intervention and Event Lowering in Diabetes Study (FIELD) | Found that fenofibrate: Decreased the requirement for the first laser and the development of diabetic macular edema Decreased the need for laser treatment compared to the control group (3.4% vs 4.9%; P = 0.0002) Appeared to have protective effects independent of blood glucose, blood pressure, and baseline lipid values |
ACCORD-Eye Study | The addition of fenofibrate to basal statin therapy resulted in: A decrease in the progression of DR, in a similar manner to that observed with intensifying blood glucose control, but with a good safety profile without increasing the risk of hypoglycemia Questions regarding fenofibrate’s mechanism of action and the pathogenesis of DR/DME |
6.2.1 Diabetes Mellitus
The duration over which the patients have had diabetes mellitus (DM) and the average degree of glucose control are the major determinants of both the development and progression of DR [17]. The Diabetes Control and Complications Trial (DCCT) showed that the benefits of “tight” glycemic control on the progression of DR took at least 2 years to become manifest, but once established, these benefits persisted for years [16]. Patients with type 1 DM in the DCCT that received standard diabetes care had average hemoglobin (Hb) A1c concentrations of 9 mg/100 ml, whereas those receiving intensive care had average HbA1c concentrations of 7 mg/100 ml. The UK Prospective Diabetes Study (UKPDS) demonstrated that improved glucose control in patients with type 2 DM significantly decreases the progression of DR [74]. By routinely asking patients about their HbA1c levels, ophthalmologists and their staffs reinforce the notion that glucose control is the most important modifiable risk factor for DR. Though lower HbA1c concentrations can further reduce the risk of DR development and progression, levels below 7 mg/100 ml can be difficult to achieve in patients with type 2 diabetes, are associated with higher frequencies of symptomatic hypoglycemia in patients receiving exogenous insulin, and may be associated with an increased risk of cardiovascular events and mortality [27].
Eyes with vision-threatening DME may benefit from improved glucose control since lowered HbA1c levels can delay or even prevent the need for ophthalmic interventions. The DCCT showed, however, that DME can suddenly and dramatically worsen in some patients when glucose control is improved, so these patients should be monitored carefully when tighter glucose control is being implemented. Patients should be warned that tighter glucose control can worsen DME but that the ocular benefits of tighter control accrue after 2 years.
Recent data suggest that improved control of modifiable systemic risk factors may reverse DME. In the RISE/RIDE trials, 18% of patients in the sham group had visual improvement of at least +15 letters, and 30% of patients in the sham arm never received “rescue” laser [8]. For patients with DME treated with anti-VEGF therapy, the composition of the anti-glycemic regimen (insulin vs. oral hypoglycemic) does not appear to influence the efficacy of the ocular therapy [46].
Prior to the introduction of pharmacotherapy, patients with center-threatening DME and poor glucose control would routinely receive prompt macular photocoagulation [23] because laser could not be counted on to improve VA if the edema spread to the fovea and adversely affected vision. These same eyes can now be observed while glucose control is improving, because expanding edema with worsening VA can probably be reversed by initiating pharmacotherapy.
Diabetic macular edema has been attributed to pioglitazone (Actos) and rosiglitazone (Avandia) [64], but the ACCORD trial failed to find an increase in VA loss in patients receiving thiazolidinediones [70]. Some investigators speculate that DME development due to these drugs is idiosyncratic and that ophthalmologists should consider asking that they be discontinued if a patient develops suspicious DME.
6.2.2 Systemic Arterial Hypertension
Systemic arterial hypertension (SAH) potentiates hyperglycemia-induced damage to capillary endothelial cells and results in blood-retinal barrier breakdown. Adequate blood pressure control (systolic pressure between 130 mmHg and 150 mmHg) decreases the adverse effects of SAH on DR [34, 37, 74], but tighter control may not provide additional advantage [27]. Angiotensin-converting enzyme (ACE) inhibitors should be considered to delay both the onset and progression of DR [24, 39]. Some evidence suggests that ACE inhibitors reduce the likelihood of developing DR in diabetic patients who are not hypertensive [39]. Losartan and candesartan reduce the progression of DR in diabetic patients with SAH [39, 66].
6.2.3 Hyperlipidemia
Elevated blood lipid concentrations potentiate blood-retinal barrier breakdown and sometimes leads to the accumulation of lipid precipitates in the retina (lipemia retinalis). Though the incidence of lipemia retinalis may be dropping, large lipid deposits within the fovea can damage photoreceptors and irreversibly decrease vision. Lowering serum triglyceride levels with statin drugs decreases vision loss in patients with DR. Some investigators also recommend adding fenofibrate [70] because it further lowers serum lipid concentrations and may decrease vision loss by another unidentified mechanism [33].
6.2.4 Sleep Apnea
Obstructive sleep apnea (OSA) has been associated with DME, but treatment of OSA has not been shown to alter the course of DME. Individual cases of DME resolution after successful treatment of OSA have been reported [29].
6.3 Screening Guidelines
Diabetic retinopathy screening guidelines have been published by the American Academy of Pediatrics (AAP) [2, 44], the American Academy of Ophthalmology (AAO) [1], the American Diabetes Association (ADA) [3], and the Canadian Ophthalmological Society (COS) [10]. Guidelines vary slightly among the organizations, but the overall recommendations are remarkably similar for patients with type 1 DM. The AAP recommends that ophthalmologic examinations begin “3–5 years after DM is diagnosed if the patient is >9 years of age” followed by annual examinations [2]. The AAO Preferred Practice Pattern recommends that ophthalmologic exams begin “3–5 years after DM is diagnosed” followed by annual examinations [4]. The ADA Position Statement recommends the first eye exam “within 3–5 years after diagnosis of diabetes once the patient is 10 years of age or older” followed by yearly examinations [3]. The Canadian Ophthalmological Society recommends that screening for DR should begin “5 years following the diagnosis of diabetes” or at puberty followed by yearly examinations [10]. For patients with type 2 DM, the initial ophthalmic examination should be performed shortly after diagnosis, and this should be followed by yearly exams.
These guidelines are based on data accumulated from several studies, most of which found that DR develops between 8 and 10 years after DM is diagnosed [38, 45, 75]. Some patients, however, develop proliferative diabetic retinopathy (PDR) and peripheral neuropathy within 1–2 years of diagnosis [22, 30] despite excellent glucose control. No cases of PDR have been discovered in the first decade of life, but hormonal changes associated with puberty may increase the risk of developing DR during the second decade [22, 30, 37, 44]. For these reasons, the guidelines generally recommend that screening for patients with type 1 DM begin 3–5 years after diagnosis, once the patient is either 10 years old or has reached puberty – whichever occurs first.
Adherence to DR screening guidelines, unfortunately, is generally very poor. A study of 902 patients with type 1 DM reported that 28% had never received an eye examination and 11% of them were at high risk of vision loss [77]. A study from a tertiary care pediatric clinic reported that only 35% of diabetic children between the ages of 15 and 20 years had been referred for an eye exam [63]. During the first year after screening guidelines for DR were implemented in Australia, no change in practice patterns were noted as only 60% of at risk patients received an eye examination in 2010 [48]. A database claims study pegged the annual ophthalmic examination rate at 34%, with only 16% of patients receiving examinations during consecutive years [51].
Diabetic retinopathy can progress rapidly during pregnancy, presumably due to elevated estrogen levels and the presence of an insulin-like growth factor [36]. Patients who develop gestational diabetes are not at risk of developing DR, but if a patient with diabetic retinopathy becomes pregnant, monthly examinations should be performed until delivery.
6.3.1 Screening Methods
Color fundus photographs remain the gold standard for detecting DR, but routine fundus photos have not been proven necessary if patients receive dilated fundus examinations. Photographic screening programs have become particularly important in developing countries where an insufficient supply of physicians and long travel distances preclude the performance of regular dilated fundus examinations [55]. Telemedicine DR screening programs can be created with modestly priced standard or non-mydriatic fundus cameras, and office personnel can be trained to take high-quality fundus photographs. These programs can generate high-quality photographs that enable readers to rule out vision-threatening retinopathy with a high degree of certainty. Adequate fundus images can be obtained from 85% of patients in rural settings even with the use of non-mydriatic cameras [12]. The value of these screening programs stems from the high DR detection rates – 12 to 29% in some programs.
Recently developed adapters can transform nearly any cellular telephone into a high-quality fundus camera [61]. The low cost of the adapters together with the widespread availability of cellular telephones allows the placement of inexpensive fundus cameras into nearly any office. With this technology, patients may receive high-quality retinopathy screening evaluations in their primary physicians’ offices, after which photos can be electronically transmitted to ophthalmologists’ offices or reading centers for evaluation. Patients with high-risk retinopathy can be referred for complete ophthalmology examinations or scheduled for future photographs. Issues regarding Health Insurance Portability and Accountability Act (HIPAA) compliance and insurance billing can be challenging, but systems that address these concerns have been developed.
Some reading centers employ specially trained technicians to grade clinical photographs, whereas others use ophthalmologists and retina specialists. As screening programs expand and include more patients, the number of transmitted photographs may overwhelm the ability of reading centers to properly evaluate them. Computer programs are being developed to efficiently identify abnormal digital photos, make accurate diagnoses, determine the risk of vision loss, and either recommend referral to an ophthalmologist or defer for future screening [32, 76]. Developing and validating such software is a complicated task, and powerful hardware is needed to read thousands of photographs. Software developers in several countries are developing programs that may be commercially available within 5 years.
6.4 Imaging
High-resolution, stereoscopic fundus photography remains the most accurate way to assess the presence and severity of diabetic retinopathy. Diabetic seven-field photographic surveys remain the standard for assessing DR severity in both the macula and mid-periphery (Fig. 6.2), but newly available ultra-widefield photography is easy to perform, can be done through undilated pupils, and often provides excellent views of both the macula and peripheral retina. Stereoscopic macular photographs can identify the degree and extent of macular thickening, but spectral domain optical coherence tomography (SD-OCT) is widely available, is easier to perform, and provides quantitative measurements for serial comparisons.
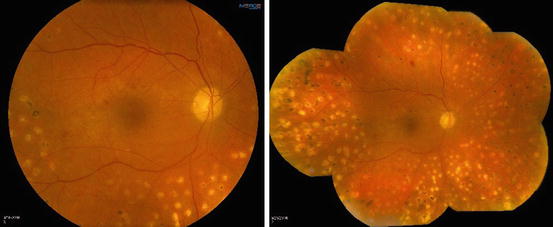

Fig. 6.2
This composite photograph compares a single 50° photograph (left) to a seven-field composite (right). Note the better view of the mid-peripheral retina with the seven-field composite
Fluorescein angiography identifies perfusion defects in the retinal vasculature and helps surgeons plan laser photocoagulation sessions. Laser treatments can be directed at leaking microaneurysms and capillary beds [56], and angiography is necessary if treatment with the NAVILAS system is being planned [41]. Angiography also identifies areas of perifoveal capillary non-perfusion, which should be avoided during macular laser photocoagulation. Identifying macular non-perfusion helps set reasonable expectations for visual recovery. Capillary non-perfusion of the macula is not a contraindication to pharmacotherapy, but visual outcomes in these eyes are usually poorer despite satisfactory resolution of edema.
Persistent retinal neovascularization can cause repeated vitreous hemorrhages even after the placement of what appears to be adequate PRP. Persistent peripheral NV can best be detected with ultra-widefield angiography [35], which can guide the placement of additional PRP.
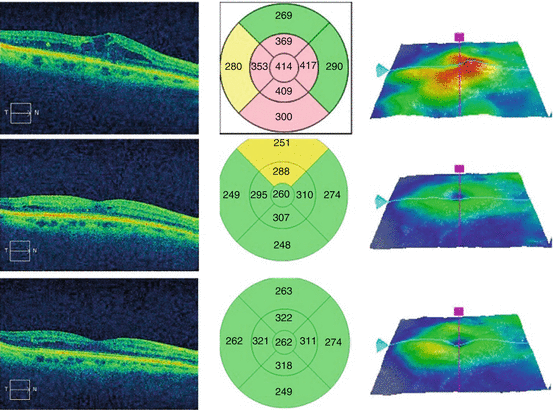
Spectral domain optical coherence tomography (OCT) imaging of the macula has become indispensable for evaluating DME (Fig. 6.3). OCT produces detailed cross-sectional images with accurate thickness and volume measurements [18]. The pivotal phase III drug trials used OCT imaging to include and exclude patients. Furthermore, OCT measurements are the best way to monitor the effectiveness of treatment and determine the need for retreatment or switching therapy.
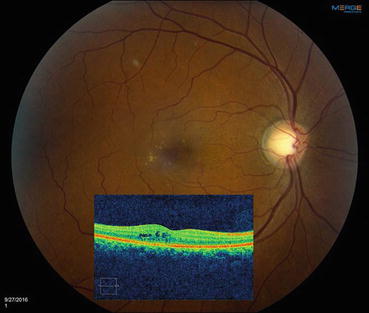

Fig. 6.3
This composite figure shows a color fundus photograph of an eye with diabetic macular edema with a superimposed horizontal optical coherence tomography (OCT) scan through the macula. The photograph shows scattered microaneurysms and hard exudates in the temporal macula, but retinal thickening can only be appreciated with the OCT
OCT angiography (OCTA) is a recently introduced technology that visualizes patent retinal and choroidal blood vessels without the use of an injected dye. OCTA detects perfusion defects in both the superficial and deep retinal capillary plexuses and may ultimately replace fluorescein angiography for evaluating retinal vascular disease.
6.5 Treatment of Diabetic Macular Edema
6.5.1 Non-Center-Involving Diabetic Macular Edema
A decision tree for the treatment of DME is shown in Fig. 6.4.
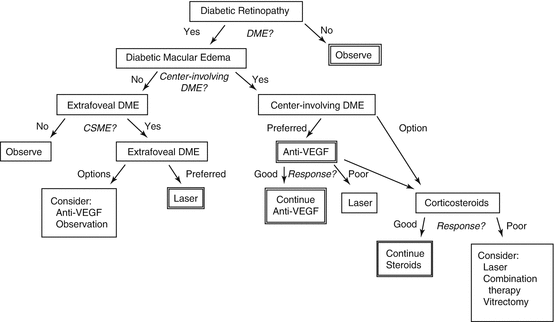

Fig. 6.4
This figure shows a paradigm that can be used to guide treatment for most eyes with diabetic macular edema. Due to the complexity of the disease and the variability of responses, direct application of this paradigm may not be possible in all cases. DME diabetic macular edema, VEGF vascular endothelial growth factor, CSME clinically significant macular edema
Eyes with non-center-involving DME usually have excellent visual acuity unless the foveal avascular zone is affected by capillary dropout. When evaluating these eyes, physicians must decide if treatment is indicated or if the eye can be carefully followed. None of the pivotal pharmacotherapy registration trials enrolled patients with non-center-involving DME, so evidence-based treatment guidelines from the pharmacotherapy era are lacking. Before ocular pharmacotherapy became available, preventing foveal thickening and its associated loss of VA was critically important, but now that pharmacotherapy can resolve center-involving edema and reverse mild visual deficits in most cases, many of these eyes can be followed until edema affects the fovea.
Since publication of the ETDRS results, macular laser photocoagulation was performed when eyes developed clinically significant macular edema (CSME) [23]. Clinically significant macular edema no longer dictates the need for treatment in the pharmacotherapy era, but it helps identify eyes with non-center-involving macular edema that threatens the fovea. Eyes with hard exudates within 500 μm of the fovea and adjacent macular thickening, or with an area of macular thickening 1 disc diameter (DD) in size any part of which is within 1 DD of the fovea, are at increased risk of vision loss over the next 3 years and should be watched carefully. When considering treatment of eyes with CSME, physicians should remember that this diagnosis was based on contact lens biomicroscopy and not SD-OCT scanning.
Many physicians perform laser photocoagulation to eyes with non-center-involving CSME to avoid beginning a series of anti-VEGF injections. Low-intensity laser photocoagulation of microaneurysms and areas of capillary leakage [54] (as discussed in Chap. 3) frequently resolves edema and prevents or delays progression to center-involving edema.
6.5.2 Center-Involving Diabetic Macular Edema
The pivotal phase III drug trials demonstrated that anti-VEGF therapy with ranibizumab or aflibercept improves VA and resolves center-involving edema better than laser photocoagulation. Patients enrolled in these trials had best corrected visual acuity (BCVA) measurements that ranged from 20/40 to 20/320 [40, 53], but eyes with center-involving DME and BCVA better than 20/40 have not been rigorously compared to laser in pharmacotherapeutic trials. Therefore, firm treatment guidelines for eyes with center-involving DME but good VA are not yet available. The ongoing DRCR.net Protocol V is comparing laser photocoagulation with intravitreal aflibercept for eyes with BCVA better than 20/32 [73]. Treating these eyes with anti-VEGF injections is a reasonable strategy since this rapidly resolves edema, improves vision, and avoids complications resulting from laser photocoagulation. If we extrapolate from DRCR.net Protocol T, each of the 3 anti-VEGF drugs should work equally well for this set of patients [21]. Focal or grid-pattern laser photocoagulation also remains a reasonable alternative for these eyes, particularly if patients are still asymptomatic. Since the visual acuity in these eyes is already good, laser is being performed primarily to prevent loss of vision.
The RESTORE trial showed that for eyes with CRT <400 μm, visual improvements are the same whether treated with laser or ranibizumab [50]. These results convinced the National Institute for Health and Care Excellence (NICE) of the UK Department of Health to have physicians use ranibizumab for eyes with CST >400 μm but not for those with CST <400 μm [62]. Post hoc analyses of other phase III trials failed to uncover similar results, and surgeons in other countries generally use anti-VEGF drugs as first-line therapy in eyes with CMT between 300 and 400 μm. When caring for a patient with good VA, however, one must remember that treatment should be based on the patient’s complaints and wishes, and not solely on the appearance of the OCT.
Eyes with center-involving DME and BCVA of 20/32 or worse most closely resemble those enrolled in the phase III registration trials for ranibizumab and aflibercept [40, 53]. Trials with off-label bevacizumab have provided level II data with which to base clinical decisions [49, 65]. The National Eye Institute-sponsored DRCR.net Protocol T trial showed that for eyes with baseline BCVA of 20/32 to 20/40, each of the anti-VEGF drugs produces VA gains of approximately +8 letters [21]. But for eyes with baseline BCVA of 20/50 or worse, aflibercept (+18.9 letters) produces greater gains in VA than ranibizumab (+14.2 letters) and bevacizumab (+11.8 letters) at 1 year. Aflibercept also produces greater macular thinning (−169 μm) than either ranibizumab (−147 μm) or bevacizumab (−101 μm). On average, patients received fewer aflibercept (9) than ranibizumab (10) or bevacizumab (10) injections. Unfortunately, Protocol T used a complicated retreatment algorithm that is difficult for most practices to follow. The simplified version of the Protocol T algorithm says that patients should receive 5 monthly injections followed by continued injections until stable. The 2-year data has been presented but not yet published in peer-review journals. Gains in BCVA were maintained for all groups, but for patients with baseline BCVA of 20/50 or worse, the difference between the aflibercept and ranibizumab groups decreased to 2 letters and was no longer statistically significant.
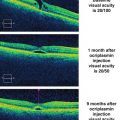
The pivotal phase III anti-VEGF trials evaluated the efficacy of monthly (ranibizumab and aflibercept) or bimonthly (aflibercept) injections on center-involving DME and patients were switched to PRN ranibizumab after 12 months in RESTORE and after 36 months in RISE/RIDE. Aggressive treatment with monthly injections probably produces the best possible visual results (Fig. 6.5), but these regimens are expensive and compliance is difficult to maintain. In contrast, DRCR.net Protocols I and T featured monthly injections for 4 months or until dry, before switching to monthly PRN injections that are based on retreatment criteria that many physicians believe are too complex to use in most clinical settings. Compared to monthly injections, as-needed treatment regimens reduce the number of injections but not the number of clinic visits.