Chapter 43 Cryptococcosis
Disseminated cryptococcosis has been the most common life-threatening fungal infection in patients with acquired immunodeficiency syndrome (AIDS), affecting up to 8% of patients with advanced human immunodeficiency virus (HIV) infection.1–3 In limited resource settings such as sub-Saharan Africa, the prevalence may be much higher and in one study accounted for 17% of all deaths in an AIDS cohort in Uganda.3a The most common manifestation is meningoencephalitis, although disseminated disease is well-described, and localized infection of many organs can occur. Carefully conducted clinical trials have helped define effective therapy for this infection, although many issues in management remain unresolved. This chapter reviews the microbiology, pathogenesis, epidemiology, clinical syndromes, and treatment of cryptococcosis in patients with AIDS.
MICROBIOLOGY
There are more than 20 known species of Cryptococcus,4 but C. neoformans is essentially the only human pathogen, although there have been isolated case reports of infection with Cryptococcus albidus and Cryptococcus laurenti. C. neoformans is an encapsulated, round to oval yeast that reproduces by narrow-based budding. It has a surrounding polysaccharide capsule ranging from 11 mm to more than 301 mm when cultivated in the laboratory.5 In its natural environment, it is smaller and poorly encapsulated. Mycelia are produced bearing basidiospores ranging from 1 to 81 mm in its perfect state (Filobasidiella neoformans). F. neoformans has never been isolated from patients or in nature.6 During the exponential growth phase of Cryptococcus, the generation or doubling time ranges from 2.5 to 6.0 h.
There are two pathogenic varieties, C. neoformans var neoformans and C. neoformans var gattii, which can be distinguished on the basis of capsular serotypes.5,7 These two varieties differ in geographic distribution; but their growth requirements for temperature, phenol oxidase production, and capsule formation are similar. They can also be distinguished by growth characteristics on canavanine-glycine-bromthymol blue agar. The serotypes of C. neoformans are designated A, B, C, and D based on antigenic determinants on the polysaccharide capsule.8 Serotypes A and D (C. neoformans var neoformans) are the most common cause of infection and are most often seen in immunocompromised hosts. Serotypes B and C (C. neoformans var gattii) are endemic in Australia and southern California, are usually isolated from normal hosts, and have a predilection for invading the central nervous system (CNS).9,10 Although the incidence of cryptococcosis in AIDS patients appears to vary geographically, even in areas where var gattii is endemic, most of the isolates from patients with AIDS are var neoformans.11,12 The genotypic features of C. neoformans were actively pursued during the 1990s, now with more than 20 genes cloned and sequenced.13–27 Electrophoretic karyotyping has identified 12 genes in C. neoformans var neoformans compared to 13 genes in C. neoformans var gattii.14
PATHOGENESIS AND HOST DEFENSE MECHANISMS
It is postulated that initial infection occurs via inhalation of the basidiospores or unencapsulated forms, leading to subsequent colonization of the airways and subsequent respiratory infection.8,28 C. neoformans has been isolated from the nasopharynx of ∼50% of AIDS patients with cryptococcosis, whereas C. neoformans has not been isolated from AIDS patients without cryptococcosis.29 The initial immune response correlates best with polymorphonuclear leukocyte activity more than macrophage activity. However, the role of pulmonary macrophages is paramount in the host’s control of the yeast inoculum,30 and complement-mediated phagocytosis appears to be the primary initial defense against cryptococcal invasion.5 Other host–yeast interactions, such as the role of CD4+ T-lymphocyte and CD8 + T cells, as well as the role of cytokines, also appear to be important.30–33 It is clear that the absence of an intact cell-mediated response results in ineffective ingestion and killing of the organism, leading to dissemination and increased cryptococcal burden. In murine models both CD4+ T-lymphocyte and CD8+ T cells are required to inhibit cryptococcosis. Natural killer (NK) cells also appear to have a limited role.30,34 Specifically, interleukin-2 (IL-2)-activated T and NK cells can inhibit C. neoformans.35 The role of humoral immunity in the control of cryptococcal infections is controversial. In vitro studies of antibodies to the soluble capsular polysaccharide of C. neoformans have revealed enhanced phagocytosis, increased fungicidal activity of leukocytes, and increased fungistatic activity of NK cells.36–39 Animal models of both polyclonal and monoclonal antibody immunization have had varying results.40–43
Factors associated with the virulence of C. neoformans include its polysaccharide capsule, production of melanin, the mating type, and growth at 37°C (thermotolerance).44–46 The polysaccharide capsule, composed mainly of glucuronoxylomannan, was the first C. neoformans virulence factor associated with disease.47 There is also some evidence of an interaction between C. neoformans and HIV-1 in vitro. Soluble C. neoformans capsular polysaccharide is able to enhance HIV infection in cultured cells, subsequent production of HIV-1, and in vitro production of syncytia.48 Additionally, a study of dual immunocytochemical staining for HIV-1 and cryptococcal antigen in brain tissue of AIDS patients showing anatomic co-localization of both pathogens suggests a possible synergistic effect of virus and yeast in vivo.49
C. neoformans is distinguished from other yeasts by its ability to assimilate urea and its possession of membrane-bound phenol oxidase enzymes, which are able to convert phenolic compounds into melanin. C. neoformans strains that produce melanin are more virulent in mouse models than strains that do not produce melanin.38,39 In addition, murine cells with melanin appear more resistant to phagocytosis.45 It is postulated that the propensity of C. neoformans to invade the CNS may be due to its ability to synthesize melanin from catecholamines, which are present in large concentrations there.49
EPIDEMIOLOGY
In the developed world prior to the use of more effective antiretroviral therapy (ART), ∼5–10% of patients with AIDS developed cryptococcal meningitis. In 1991 the annual prevalence of cryptococcosis among HIV-infected patients in New York was estimated to be 6.1–8.5%.50 There is considerable geographic variation in the prevalence of cryptococcal infection. In the United States it is more common in residents east of the Mississippi river.51 The incidence of cryptococcosis among AIDS patients is higher in Africa and Southeast Asia than the United States, whereas it appears less often in Europe.9 The male/female ratio among AIDS patients is essentially 1:1. Cryptococcosis in children with AIDS is less common, with a prevalence rate of ∼1.4%.52 The clinical and laboratory characteristics of cryptococcosis in children is comparable to those seen in adults.
More than three-fourths of the cases associated with AIDS develop when the CD4 T-lymphocyte count falls below 50 cells/mL.51 Cryptococcosis is the initial AIDS-defining illness in 50–60% of patients and thus tends to be seen more commonly in patients in whom HIV infection has not yet been diagnosed. Risk factors that have been suggested for the development of cryptococcosis include black race, injection drug use, cigarette smoking, and several environmental exposures (presumed areas where pigeon droppings accumulate). No differences in demographic variables, HIV risk factors, or stage of AIDS was found in a case-controlled study. It is of note that the investigators were unable to detect an increased risk of the development of cryptococcal meningitis in patients who had received short and episodic courses of steroids.53
The annual incidence of invasive C. neoformans in the United States has declined in HIV-infected patients during the years since 1990. Between 1987 and 1992, cryptococcosis decreased from sixth to ninth place in rank order for infections associated with death in HIV-infected persons.3 This epidemiologic trend preceded the use of highly active antiretroviral therapy (HAART) and has been temporally associated with the increased use of fluconazole since its licensure in 1990.54 It is of note that in a large prospective, population-based surveillance study patients who had received fluconazole were significantly less likely to develop cryptococcosis, and decreases in the incidence of cryptococcosis from 1992 to 1994 were attributed to the increased use of azoles.55 A more recent review of the Kaiser Permanente medical record database from 1981 to 2000 reported similar findings whereas the highest incidence of cryptococcosis in men occurred from 1981 to 1985 and in women from 1986 to 1990.55a
A 56.5% decrease in the incidence of cryptococcal meningitis was reported between 1996 and 1997 at San Francisco general hospital, correlating with a 30% increased use of potent ART that included a protease inhibitor (PI).56 However, the decrease in incidence of crytococcosis was even more dramatic when one compared the 1995 incidence with 1996 prior to the introduction of PIs. This suggests that patients may have been receiving benefit from the use of nucleosides alone as well as the use of azoles. Furthermore, the incidence of cryptococcal meningitis had increased 30% between 1997 and 1998 and has remained constant, occurring primarily in individuals not taking potent ART.57
CLINICAL FEATURES AND DIAGNOSIS
CNS Disease
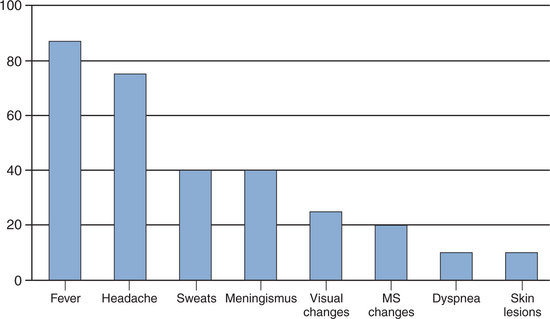
The most common manifestation of cryptococcosis is meningoencephalitis. The time span between the appearance of symptoms and diagnosis ranges from days to months.58 The clinical presentation may be nonspecific (Fig. 43-1), although infection typically presents as a subacute process.59 Symptoms may range from headache, nausea, irritability, and decline in cognitive function to frank obtundation. Physical findings may include fever and abnormal neurologic examination, with cranial nerve palsies, hyperreflexia, and papilledema. Complications of CNS infection include hydrocephalus, motor or sensory deficits, cerebellar dysfunction, seizures, and dementia. Prompt inclusion of C. neoformans in the differential diagnosis of acute and chronic meningitis for susceptible hosts should allow for prompt brain imaging study followed by lumbar puncture once a mass lesion has been ruled out. Although focal lesions caused by intracerebral granulomas (cryptococcomas) may be seen on computed tomography (CT) or magnetic resonance imaging (MRI), they are uncommon in patients with AIDS.60 Acute cryptococcal meningitis in patients with AIDS is often complicated by cerebral edema. Assessment should include measuring the opening pressure by manometry in all patients. The opening pressure is usually higher than 25 cm H2O in 60% of patients.61 Abnormal findings in the composition of cerebrospinal fluid (CSF) include a lymphocytic pleocytosis, elevated protein, and low glucose.59 However, as many as 20% of patients have no clear abnormality in the CSF profile despite positive culturesfor C. neoformans. Therefore finding a normal CSF should not exclude the possibility of cryptococcal infection. Detection of CSF cryptococcal antigen by rapid diagnostic testing should prompt initiation of therapy.
Pulmonary Cryptococcosis
Although the lung is most likely the entry portal, isolated pulmonary cryptococcosis is diagnosed less frequently than meningitis in AIDS patients.62,63 However, pulmonary involvement in the presence of disseminated disease is common. More than 10% of patients with disseminated disease may have acute respiratory failure.64 It is unclear if disseminated disease always represents progression of pulmonary disease because many patients have no evidence of pulmonary involvement at the time of diagnosis of disseminated disease. Given the relatively nonspecific clinical signs and symptoms, variable radiographic signs, and increased frequency of other pulmonary opportunistic infections, it is likely that cryptococcal pneumonia is underdiagnosed and not recognized until dissemination. In a retrospective study aimed at determining the etiology of pulmonary symptoms in HIV-positive patients with cryptococcal meningitis, 14 of 18 patients (78%) reported respiratory symptoms within 4 months prior to the diagnosis of cryptococcal meningitis.65 Other reviews suggest that 63% to 90% of HIV-infected patients with pulmonary cryptococcosis have concomitant extrapulmonary disease.62,66 However, pulmonary disease may not always be cryptococcal in origin; ∼15% of HIV-infected patients with proven cryptococcal disease have a second pulmonary pathogen, such as Pneumocystis carinii, Mycobacterium tuberculosis, or nontuberculous mycobacteria.62,67
In a retrospective chart review of 210 patients with cryptococcal disease,64 independent variables predictive of acute respiratory failure included black race, lactacte dehydrogenase level of 1500 IU/L, the presence of interstitial infiltrates, and the presence of cutaneous lesions. Mortality was 100% for the 19 patients in whom the respiratory failure could be attributed solely to cryptococcosis.
Patients with pulmonary cryptococcosis may present with cough, fever, malaise, shortness of breath, pleuritic pain, and an abnormal chest radiograph. Chest radiographs typically reveal focal or diffuse infiltrates similar to other opportunistic pathogens, particularly Pneumocystis carinii pneumonia (PCP). Other chest radiographic findings not as commonly seen include solitary subpleural nodules, mass-like infiltrates with consolidation, hilar and mediastinal adenopathy, and pleural effusions. Rarely, cavitation and empyema have been reported.68
Patients with apparently isolated cryptococcal pneumonia should be evaluated for cryptococcal meningitis with a lumbar puncture even in the absence of neurologic signs or symptoms. In addition, antifungal therapy should be initiated because early treatment of localized pulmonary cryptococcosis can be effective and can prevent dissemination.69
Other Organ Involvement
Cryptococcosis in HIV-positive patients is typically disseminated; and up to three-fourths of patients with meningitis have positive blood cultures. A small percentage of patients with cryptococcosis present initially with extrapulmonary, nonmeningeal disease, although many are subsequently found to have meningitis. Infections of the skin, joints, eye, adrenal glands, gastrointestinal tract, liver, pancreas, peritoneum, heart, prostate, and urinary tract have been described.70–77 The prostate can serve as a reservoir of infection and is a potential source of reinfection after completion of therapy.78 Cutaneous cryptococcosis is usually a sign of dissemination but may precede evidence of disease elsewhere by several weeks.70,79 The lesions vary greatly and mimic many other dermatologic entities. The most typical have an appearance resembling molluscum contagiosum,80 but cryptococcal skin disease may present as pustules, vesicles, plaques, abscesses, cellulitis, purpura, draining sinus, or subcutaneous swelling.81
Immune Reconstitution Inflammatory Syndrome
Unlike infections with Mycobacterium avium complex and cytomegalovirus, there had been few reported cases of unusual manifestations of cryptococcal disease associated with HAART. One report82 suggested that three patients had partial immune reconstitution unmasking latent cryptococcal therapy but it is unclear if these cases truly represented immune reconstitution. Two of the patients developed cryptococcal meningitis shortly after starting HAART; both patients had nadir CD4 T-lymphocyte counts less than 50 cells/μL and CD4+ T-lymphocyte counts less than 150 cells/μL after “therapy” at the time they were diagnosed with cryptococcal meningitis. The CSF analyses were typical of those seen with cryptococcal meningitis. A third patient with a recent history of cryptococcal meningitis on itraconazole developed aseptic meningitis with leukocytosis 10 days after starting HAART. This patient’s therapy was not altered, and the meningeal symptoms resolved spontaneously. There had also been two reports of cryptococcal lymphadenitis in patients receiving potent ART.83,84
More recently, there have been several larger case series reporting significant complications of cryptococcal-related immune reconstitution inflammatory syndrome (IRIS). Investigators from Dallas, Texas described four cases of IRIS and included a literature review of 21 additional cases.84a The presentations included 14 with lymphadenitis, 10 CNS complications characterized by meningitis in six and mass lesions in four as well as one pulmonary cavitary lesion. The median CD4 T-lymphocyte count at time of cryptococcal diagnosis and prior to HAART was 25 cells. The median CD4 T-lymphocyte count at time of IRIS was 197 cells and the median time to development of IRIS after cryptococcal diagnosis and initiation of HAART was 11 months (range 7 weeks to 3 years) and 7 months (range <2 weeks to 22 months), respectively. In all but one patient, the clinical manifestations resolved and four patients required anti-inflammatory medications to control their signs and symptoms.
Puthanakit and colleagues in Thailand reported an IRIS incidence of 19% among 153 symptomatic HIV-infected children of which three were attributable to cryptococcal infection.84b IRIS events occurred at a median of 4 weeks (range 2–31) after HAART.
Shelburne84c and colleagues conducted a retrospective review of 84 patients with HIV associated crypotococcal disease. Of note, the authors addressed the difficulty in diagnosing what is IRIS versus relapsed disease. For the diagnosis of the culture-negative meningitis form of IRIS, all of the following criteria had to be met: (1) the patient’s condition had clinically responded to anticryptococcal therapy; (2) after the initiation of HAART, the original symptoms returned or new inflammatory symptoms developed; and (3) during the diagnostic workup for the inflammatory process, all results of cultures had to be negative, and CSF cryptococcal antigen, if still present, had to have a level that had decreased at least four-fold from the level at the initial admission to the hospital. They also included three patients who had subclinical cryptococcal disease who developed acute disease within 180 days after initiating HAART. Of the 84 patients, 59 initiated HAART and 18 (30.5%) of the 59 developed IRIS as defined above with a median time of 30 days (range 3–330 days) after HAART. Patients who developed IRIS were more likely to be antiretroviral-naive, have a higher CSF cryptocococcal antigen, higher baseline HIV viral load and to have initiated HAART within 30 days after cryptococcal diagnosis. At the time of IRIS diagnosis, these patients had higher CSF opening pressures, increased CSF WBCs and higher glucose compared with the typical presentation of acute cryptocococcal meningitis. Although there was no statistical difference in mortality at 18 months of follow-up between those who had IRIS compared with those who did not, there was a trend towards survival benefit in the IRIS group in contrast to that found by Lorthlary and colleagues.84d They conducted a retrospective chart review of 120 HIV-infected individuals with cryptococcal disease who initiated HAART. Ten patients developed IRIS within a median of 8 months (range 2–37 months) after HAART. Three of the 10 patients with IRIS died. They reported having unpreviously diagnosed HIV, CD4 T-lymphocyte count <7 and HAART within 2 months of cryptococcosis diagnosis were independently associated with risk of developing IRIS.
These reports have prompted some clinicians to avoid prescribing HAART within the first 2–3 months following the diagnosis of cryptococcosis; however, there is no prospective data comparing the timing of HAART and development of IRIS with outcomes to make specific recommendations. Studies are underway evaluating the timing of when to start HAART in the setting of acute opportunisitic infections which hopefully will provide valuable insight into this complication and its management.
Diagnostic Evaluation
Cryptococcal antigen in the CSF is produced locally in the subarachnoid space by the invading yeast and does not represent active or passive diffusion from the serum into the CNS. Soluble circulating serum cryptococcal antigen (sCRAG) has been found only in patients with substantial infection. Several commercial latex agglutination tests (LAT) kits and one enzyme-linked immunoassay (EIA) test for detection of cryptococcal antigen in serum and CSF are widely available. The detection of CRAG in serum and CSF by latex agglutination is rapid and has a sensitivity and specificity of more than 95%.85,86 Comparative performance of four LATs and one EIA commercially available test revealed high concordance of all tests for CSF specimens, with sensitivities of 93–100% and specificities of 93–98%.86 Pronase-containing latex tests reduce the number of false-positive sCRAGs.86 False-positives can also occur secondary to infection with Trichosporon beigelii, which cross-reacts with the antigen.87 In addition, false-positives have occurred secondary to residual disinfectant on laboratory test slides.88 False-negatives may occur with low cryptococcal antigen concentrations. Although serum cryptococcal antigens are positive in 95–99% of cryptococcal meningitis cases, a positive result does not indicate that CNS invasion is present. Conversely, a negative sCRAG suggests that the patient is unlikely to have CNS disease; the test may be useful for screening symptomatic patients. The utility of sCRAG in asymptomatic HIV patients as a screening tool has not been adequately studied.89 Although routine screening for cryptococcal antigen in asymptomatic patients is not recommended, if a positive titer is noted in an asymptomatic patient, therapy should be offered89 because of the risk of its progressing to disseminated disease. The CDC is conducting a study to evaluate the utility of sCRAG in limited resource setting where the access to HAART is problematic and the incidence of cryptococcal disease is much higher than in the developed world. One could perceive that it may be a useful screen for subclinical disease at the time of initiating HAART given the potential risk of unmasking latent disease; however those studies need to be completed before recommendations for its routine use can be made.
There is little value in serial measurements of CSF and sCRAG for routine management of cryptococcal meningitis.89a,90,91 CSF cryptococcal antigen titers should decrease after effective therapy. However, there is no evidence to support following the serum antigen serially, which shows no correlation with the outcome of antifungal therapy.92 Management decisions should be based on an individual clinical assessment, not relying on cryptococcal antigen titers alone. There is insufficient data in interpretation of CRAG tests which become negative and subsequently become positive again, however, it is advised to caution on the side of a recurrence and begin a diagnostic work-up for recurrent disease.
TREATMENT
The reported acute mortality with treatment in patients with AIDS is 10–25%.59,93–95 Prior to AIDS the standard therapy for cryptococcal meningitis had been to use the combination of amphotericin B and flucytosine, with a trend toward using lower doses of amphotericin B and a shorter duration of treatment.96,97 A study of HIV-negative patients conducted by the National Institute of Allergy and Infectious Diseases (NIAID)-sponsored Mycosis Study Group (MSG)96 compared4 weeks of combination therapy with a 6-week course. That study was designed to randomize patients at the end of a uniform 4-week course of combination therapy. Only 91 of 181 patients initially treated were randomized. Of these 91 patients, cure or improvement was seen in 75% of those receiving4 weeks of therapy compared with 85% of those in the 6-week cohort. There was no difference in toxicity between the two regimens. It is important to note that 31 of 80 nonrandomized patients died, giving a 17% overall acute mortality rate with this treatment for cryptococcal meningitis. The conclusions from this study were that 4 weeks of combination therapy was acceptable for patients who did not have risk factors that correlated with a high frequency of relapse.
Although there were few HIV-positive patients in the trial, one important risk factor that was predictive of relapse was immunosuppression. Thus more prolonged courses of amphotericin B and flucytosine were used initially in the AIDS epidemic. However, early reports of the experience with the amphotericin B/flucytosine regimens in patients with AIDS were not favorable. Indeed, data suggested that flucytosine was too toxic in AIDS patients and was not beneficial.59
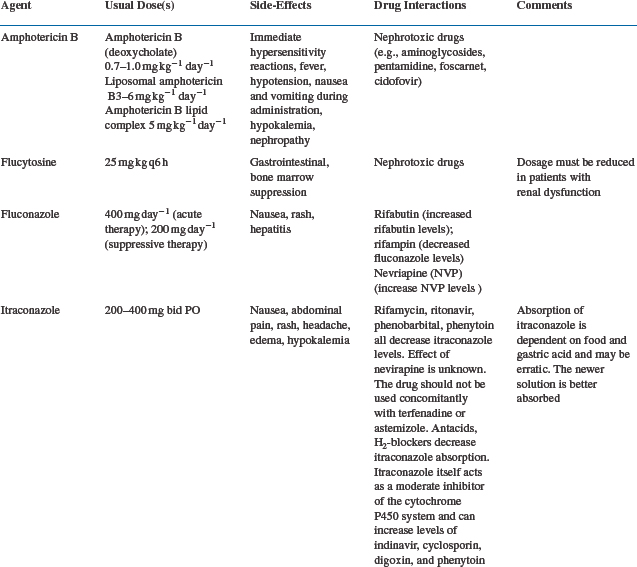
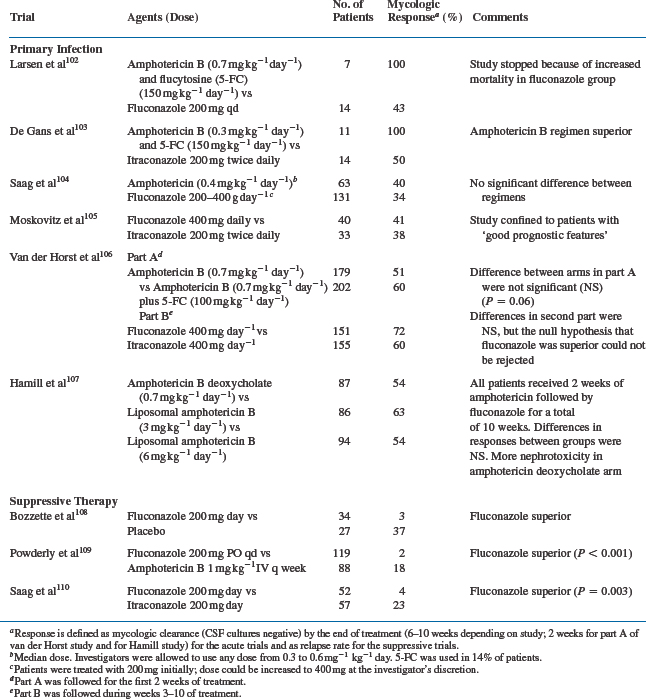
The poor outcomes and increased incidence of cryptococcal meningitis in AIDS led to a search for more effective primary treatment. This became particularly relevant with the availability of the orally active antifungal triazoles fluconazole and itraconazole (Table 43-1). Initial experience with these agents was encouraging98–101 and led to a number of controlled comparative trials (Table 43-2).
A small randomized, controlled study compared fluconazole 400 mg/day to amphotericin B with flucytosine in AIDS patients.102 Patients were initially given amphotericin B 0.7 mg kg−1 day−1 for 7 days; the frequency was then changed to three times a week for 9 weeks. Of 14 patients randomized to fluconazole, eight failed, in contrast to no failures among those given combination therapy. Of the 16 fluconazole patients, four died; there were no deaths among six patients in the combination group. The trial was terminated early because of the higher failure and mortality rates seen in the fluconazole group. Another small study compared itraconazole 200 mg bid with amphotericin B 0.3 mg kg−1 day−1 plus flucytosine 150 mg kg−1 day−1.103 A 41% failure rate was reported in the itraconazole group compared to no failures in the combination group for primary treatment. More relapses were seen in the group initially treated with itraconazole.
In contrast to these apparently superior results with amphotericin B, a randomized, prospective trial conducted by the NIAID104 comparing fluconazole to amphotericin B for treatment of acute cryptococcal meningitis in AIDS failed to show any significant differences in outcomes between the two regimens. The median dose of amphotericin B in this study was 0.4 mg/kg; flucytosine, the use of which was at the discretion of the investigators, was rarely employed. Fewer than 50% of patients in either group were successfully treated, as judged by clearance of cryptococci from their CSF cultures. However, there were hints of differences between the two regimens. Although there was no significant difference in the overall mortality between the two groups, the mortality was higher during the first 2 weeks of therapy with fluconazole. As with some other trials, the dose of amphotericin B may have been too low.
Initial therapy with azoles alone does not appear to be the most effective approach to treatment. A study comparing itraconazole 200 mg bid PO and fluconazole 400 mg daily PO as primary treatment of cryptococcal meningitis in patients with AIDS showed no statistical significance in CSF sterilization between the two groups.105 However, the study was terminated early partly because the clinical response rate was only ∼40%. Thus no prospective randomized trial of triazoles given as initial therapy to unselected patients has shown a response rate higher than 50%.
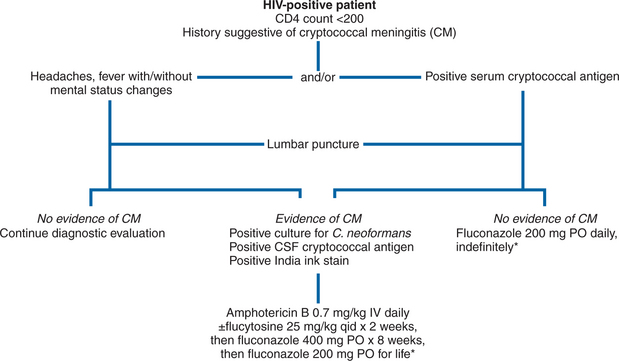
In light of these data, the NIAID’s MSG and AIDS Clinical Trials Group (ACTG) investigated a strategy of induction amphotericin B (for 2 weeks) followed by azole treatment. Patients with cryptococcal meningitis were randomized to receive 2 weeks of amphotericin B (0.7 mg kg day) with either flucytosine (25 mg/kg every 6 h) or matching placebo. The ACTG 159/MSG 017 study addressed two questions. Does adding flucytosine to amphotericin B as induction therapy for cryptococcal meningitis improve the two- or 10-week survival compared to induction with amphotericin B alone? Is itraconazole as effective as fluconazole in suppressing relapse of cryptococcal meningitis during the maintenance phase of treatment? At the end of 2 weeks, patients who were stable or improved were again randomized to receive either fluconazole 400 mg/day or itraconazole 200 mg bid. The acute mortality with this regimen was 6%, which compares favorably with anything previously reported for cryptococcal meningitis in AIDS patients.106 The addition of flucytosine to amphotericin B did not significantly improve the mortality rate or the clinical course. However, flucytosine was well-tolerated, and there was a trend to a better CSF sterilization rate with its use (60% of patients assigned to amphotericin B with flucytosine were culture-negative at 2 weeks, compared with 51% of patients who received amphotericin B alone). Furthermore, the use of flucytosine as initial therapy has been associated with a decreased risk of later relapse of cryptococcal meningitis. In the second (azole) phase of the MSG/ACTG trial, there was no significant difference in clinical symptoms, response rate, or mortality among patients randomized to either fluconazole or itraconazole, although trends favored fluconazole. An open-label Italian study of this strategic approach (high-dose amphotericin B followed by triazoles) was also associated with favorable outcomes.111 Of 31 patients treated in this fashion, 29 (94%) responded to therapy, and there were no deaths resulting from cryptococcosis. Thus it is reasonable to recommend this approach as a standard one for the treatment of acute cryptococcal meningitis in AIDS (Fig. 43-2). The Infectious Diseases Society of America (IDSA) and the US Public Health Service (USPHS) in collaboration with the HIV Medicine Association released practice guidelines endorsing this strategy. In patients unable to tolerate flucytosine, amphotericin B alone is an acceptable alternative.112,125a
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree