Most patients with corticotropin (ACTH)-dependent Cushing syndrome (CS) will have an ACTH-secreting pituitary tumor. However, approximately 10%–15% of patients with ACTH-dependent CS have an ectopic source of ACTH secretion (see Cases 58, 59, 60, 61, 62, and 63). When a clear-cut pituitary tumor is found on magnetic resonance imaging (MRI) in a woman with slowly progressive and mild-to-moderate ACTH-dependent CS, proceeding directly to transsphenoidal pituitary surgery (TSS) is a reasonable next step. However, when the pituitary appears normal on MRI, it is important to distinguish between ectopic and eutopic ACTH-dependent CS. The high-dose dexamethasone suppression test has proven to lack accuracy in making this distinction. Use of inferior petrosal sinus sampling (IPSS) to localize the source (pituitary versus ectopic) of ACTH secretion has been the most important technological advance for evaluation of CS over the past four decades. Herein we present a case in which IPSS played a key role in directing a cure of CS.
Case Report
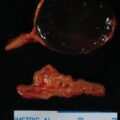
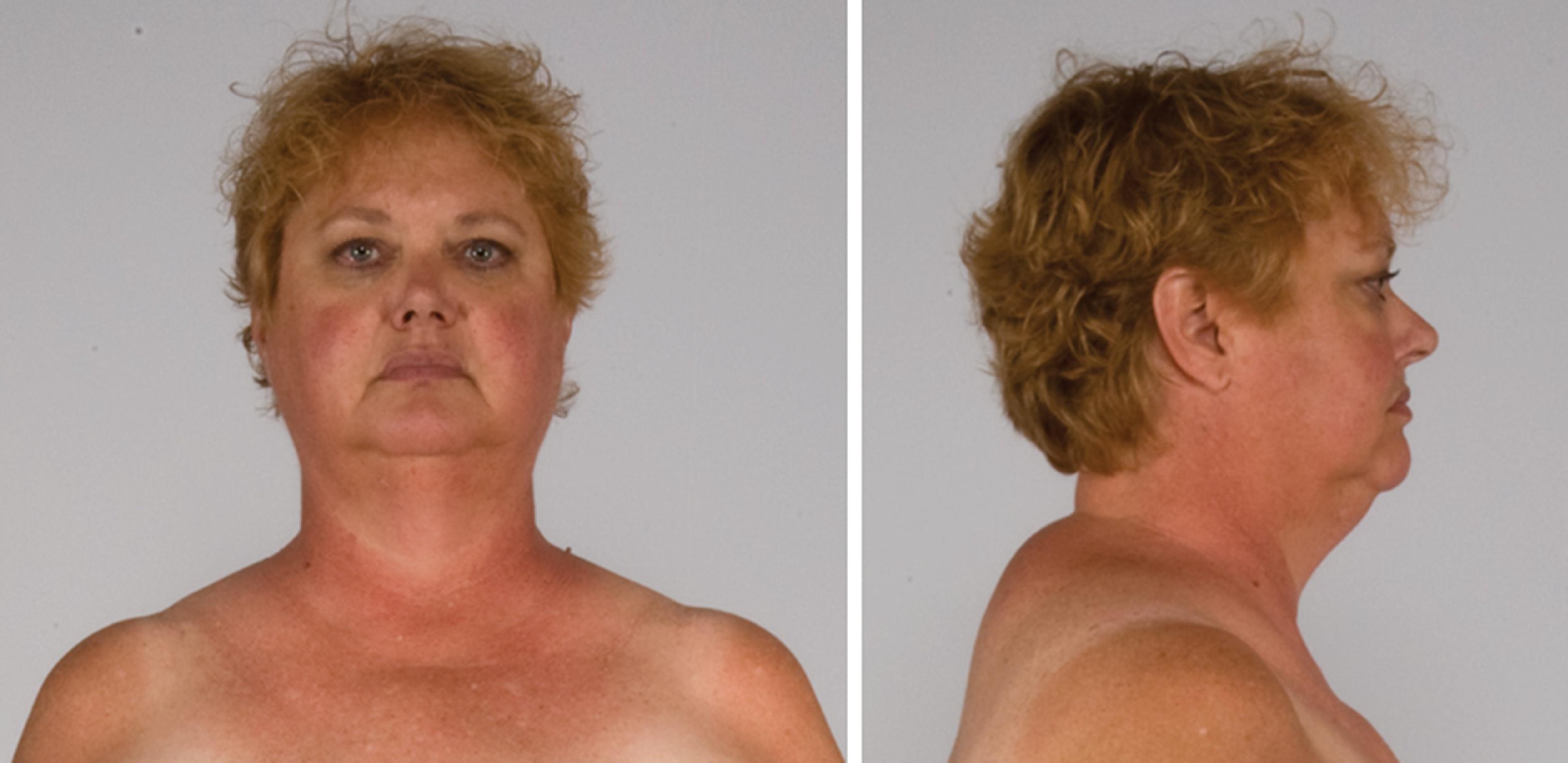
The patient was a 51-year-old woman who was referred for evaluation and management of CS. Her signs and symptoms consistent with CS included secondary amenorrhea of 2 years duration, progressive facial plethora over the past 2 years, diabetes mellitus and hypertension diagnosed 9 years previously, osteoporosis diagnosed 5 years previously, scalp hair thinning and marked curling of her hair, easy bruising, and poor wound healing. Although her weight had been fairly stable, she said “I weigh less but feel fatter.” She has lost weight from the extremities but gained abdominal girth. She had been experiencing difficulty walking up stairs. Her medications included glipizide, 10 mg twice daily; metformin, 1000 mg twice daily; sitagliptin, 100 mg daily; alendronate, 70 mg weekly; hydrochlorothiazide, 25 mg daily; and simvastatin 20 mg daily. On physical examination her body mass index was 32.2 kg/m 2 , blood pressure was 121/79 mmHg, and heart rate 80 beats per minute. She appeared cushingoid with supraclavicular and dorsocervical fat pads, a round and plethoric face, and noticeable scalp hair loss ( Fig. 55.1 ). Her extremities were relatively thin and she had proximal muscle weakness.
INVESTIGATIONS
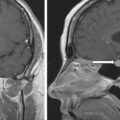
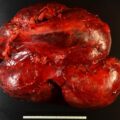
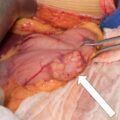
The baseline laboratory test results are shown in Table 55.1 . The serum cortisol concentrations were increased and lacked diurnal variation. The 24-hour urine free cortisol excretion and salivary cortisol concentration were more than twofold elevated above the respective upper limits of the reference ranges. The goal of laboratory testing is to build a wall of evidence to either refute or confirm the diagnosis of CS. Thus based on the clinical presentation and baseline laboratory findings, CS was confirmed. No additional testing (e.g., 1-mg overnight dexamethasone suppression test or a formal 2-day low-dose dexamethasone suppression testing) was needed. The serum ACTH concentration was high-normal and confirmed that CS was ACTH dependent. A head MRI showed a 3 × 5 × 7–mm region of relative diminished enhancement in left anterior aspect of pituitary ( Fig. 55.2 ); although somewhat heterogeneous and not classic, the findings were consistent with a microadenoma. The serum prolactin concentration was normal ( Table 55.1 ). A preoperative screening chest radiograph showed a small nodule in the left upper lung laterally. A subsequent chest computed tomography (CT) scan showed a 7-mm noncalcified nodule in the left upper lobe laterally that corresponded to the finding described on the chest radiograph ( Fig. 55.3 ). The patient was advised that her slow clinical course and mild-to-moderate degree of CS along with the apparent pituitary adenoma on head MRI were most consistent with pituitary-dependent CS. However, the nodule found on chest CT raised the possibility of an ACTH-secreting bronchial carcinoid tumor—the clinical and biochemical presentation of which can mimic pituitary-dependent CS. IPPS was advised, and the results are shown in Box 55.1 . Baseline serum cortisol concentrations on the day of IPSS confirmed active CS. The patient must have active CS on the day of IPSS—otherwise the test results are invalid and cannot be used to direct patient care. The central-to-peripheral ACTH gradient from the right IPS was 13.3-to-1 before corticotropin-releasing hormone (CRH) administration and 36.6-to-1 after CRH administration. Pituitary dependent-disease is confirmed when these gradients are >2-to-1 at baseline and >3-to-1 after CRH administration. In nearly all patients with pituitary-dependent CS the central-to-peripheral ACTH gradients markedly exceed these cutoffs (as was the case for this patient); when the gradients are just above the cutoffs, we worry that the patient may actually have ectopic CS. In addition, the peripheral CRH stimulation test showed a 90% increase in ACTH concentration from baseline and a 100% increase in cortisol concentration from baseline. The diagnosis of pituitary-dependent CS is supported by finding the CRH-stimulated increment in ACTH to be >50% from baseline and the increment in cortisol to be >20% from baseline. Thus pituitary-dependent CS was confirmed.
| Biochemical Test | Result | Repeat Test Result | Reference Range |
| Sodium, mEq/L | 141 | 135–145 | |
Potassium, mEq/L | 4.1 | 3.6–5.2 | |
Fasting plasma glucose, mg/dL | 96 | 70–100 | |
Glycosylated hemoglobin, % | 7.7 | 4–6 | |
Creatinine, mg/dL | 0.7 | 0.6–1.1 | |
eGFR, mL/min/BSA | >60 | >60 | |
8 am serum cortisol, mcg/dL | 25 | 15 | 7–25 |
4 pm serum cortisol, mcg/dL | 25 | 2–14 | |
24Hour UFC, mcg | 284 | 103 | 3.5–45 |
24-Hour urine volume, L | 1.7 | 1.3 | Goal <4 L |
Late night salivary cortisol, ng/dL | 215 | 198 | ≤100 |
ACTH, pg/mL | 52 | 50 | 10–60 |
DHEA-S, mcg/dL | 138 | 15–200 | |
Prolactin, ng/mL | 11 | 3–27 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree