72.1
Introduction
Despite the remarkable strides that scientists and health-care professionals have made in the diagnosis and treatment of osteoporosis over the last quarter-century, the battle against this metabolic bone disease is far from won. The area in which we still face critical challenges is in the use of pharmaceutical medications to treat the disease. Medications have been developed and tested, but they have not been well accepted and adhered to by many patients. This paradox has puzzled health services researchers since 1995 when the first nonhormonal treatment for osteoporosis was finally available. Many resources and much professional time have been spent trying to make the connection between these noteworthy advances in these pharmaceutical agents and the people who need them.
I will begin by briefly tracing the history of osteoporosis medications, with that key starting point of 1995. This section will illustrate the efforts made to connect dosing and delivery of these drugs to patient needs and highlight how ineffective these efforts were. I will also examine studies designed to improve adherence and see how different they are from information provided in the popular press and on the Internet to patients. Finally, I will close by emphasizing how truly important adherence to medication is and wonder why—after all this time—we have done little to improve it.
72.2
A brief history of osteoporosis pharmaceutical treatments
First, let me say that this is neither an advertisement for nor a promotion on the part of osteoporosis prescription medication. I believe in multidimensional therapy for patients diagnosed with osteoporosis, and I fully endorse exercise and diet as a part of a treatment plan for those with this disease. But for those with existing fractures or the lowest bone density, there really is not a choice if they want to treat the underlying disease. No alternatives of which we know can affect the positive changes associated with osteoporosis prescription medicines. That said, I am proposing some of the issues here because I think there are people whose osteoporosis is serious and who may benefit from pharmaceutical intervention. I hope this chapter will elucidate why many people decide not to take these medications and how the health-care community might emphasize their importance in our current battle against fractures. My goal in this chapter is to discuss ways in which to effectively reduce the risk of osteoporotic fractures.
As we try to find answers to the questions of why osteoporosis patients are not taking their osteoporosis medications, the history of these medications takes on increasing significance. In 2010 a total of 10.2 million American adults have osteoporosis diagnosed by bone density and another 43.4 million have low bone density . By 2050 a total of 85 million Americans will be 65 years and old and will represent 22.03% of the population . Imagine how many osteoporotic fractures will occur once the most vulnerable group for fractures grows that large.
72.2.1
First available were hormonal options
Nearly two centuries ago, French pathologist Jean Georges Chretien Frederic Martin Lobstein first coined the term osteoporosis to describe larger than expected holes in certain of his patients’ bones . A century later, in 1941, Albright et al. brought attention to the postmenopausal period and osteoporosis, giving us another potential cause of fragile bones and linking postmenopausal and osteoporosis . In the next year the Food and Drug Administration (FDA) approved Premarin for treating menopause and in 1972 also found estrogen potentially effective as a preventive or treatment for osteoporosis . A second hormonal drug, injectable calcitonin, was approved for the treatment of osteoporosis in 1976 . Although what caused bone loss in 1976 beyond aging and atrophy was not clear, the administration of estrogen to treat or prevent osteoporosis was a critical step in our understanding of this disease. Unfortunately, in 1976, there was no good way to determine the effectiveness of these drugs; bone density measurement was not yet available. Calcitonin and estrogen were definitely better than no treatment at all, but no empirical evidence was available to support this. However, the Women’s Health Initiative data later found that estrogen plus progestin significantly increases bone mineral density and reduces the risk of fracture in healthy postmenopausal women .
72.2.2
Oral bisphosphonates expanded choices beyond hormones
The first nonhormonal medication for osteoporosis was FDA approved in 1995. Alendronate, a bisphosphonate and antiresorptive drug, showed efficacy against osteoporosis by reducing fractures and preventing bone density loss . Unfortunately, alendronate faced two major challenges that might suggest why adherence to it would be poor over the long haul. The dosing ritual for alendronate and other oral bisphosphonates is complex [i.e., patients should take oral bisphosphonates upon arising for the day; the tablet should be swallowed with a full glass of water (6–8 oz); patients should not lie down for at least 30 minutes and should not have food or drink other than water in that same interval]. Researchers and clinicians expressed concern about the viability of patients taking a drug with such a complex dosing regimen . In an excellent review of the literature on adherence to oral bisphosphonate treatment , multiple studies showed that adherence to these medications was extremely poor and insufficient to ensure a therapeutic dose of these drugs. Thus pharmaceutical companies were challenged to find ways in which these medications could be delivered that would increase adherence.
In addition, alendronate showed a different side effect profile in general use than it had in clinical trials. De Groen et al. noted that real-world problems such as patients having comorbidities and not seeing providers nearly as frequently as they did during trials contributed to this difference. Further, patients with dyspepsia and other gastrointestinal (GI) problems were excluded from the clinical trials .
The approval of alendronate paved the way for other oral bisphosphonates. The second was risedronate, approved in 1998 . At its initial approval, risedronate was dosed as an oral tablet taken daily with dosing regimen similar to that of alendronate ; thus risedronate was likely to have similar compliance challenges as alendronate. Studies showed, however, that risedronate caused fewer GI side effects than alendronate, especially early in the medication process . Unfortunately, the difference in compliance between the two medications was minimal . Both of these agents, however, do nothing if patients do not take them as prescribed. This was simply not happening.
72.2.3
Alternative bisphosphonate dosing regimens
Concern increased about the poor adherence to these first oral bisphosphonates. Although changing the oral dosing regimen seemed impossible given the poor absorption of the tablet and the GI side effects , researchers began to examine reducing dose frequency as a solution to poor adherence . Perhaps those women who preferred having their coffee or breakfasts right after awakening might consider a once-weekly regimen. It would have the same requirements, but they would be required 1 day instead of 7 days a week.
Virtually every study of patient acceptance of weekly dosing showed that patients found the idea of weekly dosing more attractive than daily dosing. A three-country study was undertaken in which participants were divided into two groups: one in which patients were started on a weekly bisphosphonate (70 mg alendronate and 35 mg risedronate) and the other in which patients were started on a daily bisphosphonate (5 or 10 mg of alendronate or 5 mg of risedronate). In each country, study participants were significantly more compliant with weekly dosing and significantly more persistent with weekly therapy. Medication possession ratio (MPR) was used to determine percent compliant with these dosing intervals, and the weekly MPR compared to the daily was 69% versus 58% in the United States, 76% versus 64% in the United Kingdom, and 59% versus 53% in France (all P <.001). But none of these MPRs achieved the 80% compliance rate suggested as necessary for appropriate fracture reduction . Adherence was better with weekly bisphosphonates but not yet universally accepted. Risedronate was formulated as a weekly dose as well in 2007, and studies were done to see whether there were differences in adherence for the two medications’ dosing intervals. These studies showed that although adherence was better with weekly than with daily dosing, the level of adherence was still very poor. Perhaps this solved a small part of the problem, but more extended dosing—at least at this level—was not the solution.
72.2.4
Alternatives to bisphosphonates
Meanwhile, a nonbisphosphonate, raloxifene, a selective estrogen receptor modulator (SERM), was approved by the FDA in 1997. Raloxifene prevents bone loss and reduces the risk of vertebral fractures but had no effect on hip and other nonspine fractures . In addition to its indication for osteoporosis, raloxifene also reduces the risk of invasive breast cancer by 76% . Thus taking raloxifene can address two major concerns of postmenopausal women with a single pill. It was still a daily dose but required no complex dosing strategy . Raloxifene dosing has remained the same since its initial approval.
As the dosing intervals changed, few studies actually examined what patients preferred. In a study by Richards et al., nearly 2500 postmenopausal women were surveyed about which osteoporotic dosing schedule would be preferred . In this study, 45% of the older participants preferred daily therapy, 20% preferred weekly dosing, and 30% selected monthly doses. In addition, women not currently on an osteoporosis therapy preferred a medication with daily dosing that did not require fasting or remaining upright for 30 minutes. This large, population-based study suggested for the first time that patient preferences and individually tailored dosing were preferable. Such conditions for therapy described in Richards et al. were not possible at that time; they were theoretical rather than actual options . However, raloxifene dosing was more flexible than that of the bisphosphonates.
The simpler dosing regimen with raloxifene affected patient attitudes toward osteoporosis and the medication for it. In a comparison of daily alendronate and daily raloxifene compliance , investigators found that compliance with raloxifene was significantly better than that with alendronate; patient satisfaction was higher as well. Another study examining differences in adherence among daily and weekly alendronate, risedronate and raloxifene users showed the highest compliance with raloxifene in the daily dose . Also, discontinuation due to side effects was the highest in the alendronate weekly group, giving support to the concept that dosing frequency was not the only factor in patient adherence.
Treatment for osteoporosis changed dramatically again when, in November 2002, the FDA approved teriparatide, a form of parathyroid hormone (PTH) that stimulates both bone formation and bone resorption, depending on how it is administered . Although continuous dosing of PTH leads to bone loss, intermittent dosing—achieved through daily self-injections—increases bone mass. Teriparatide is an osteoanabolic and builds new bone. This new therapeutic area created a conundrum for certain patients with osteoporosis. On one hand, this new medication actually built bone and did not cause GI side effects, but having to self-inject every day seemed onerous, even though its use was limited to a maximum of 24 months. The two issues that contribute to poor adherence to osteoporosis medicines—daily dosing and an injectable medication—were both present in teriparatide.
Yet two studies of adherence to teriparatide showed unexpected outcomes. A 2009 study of adherence to teriparatide showed 90% adherence at 6 months. Second, a study by Ziller et al. of 50 postmenopausal women showed that 80% of women treated with teriparatide were adherent to their treatment. These rates of adherence were surprising and much better than rates with bisphosphonates. About 4 years later, a multicenter trial done in Italy had positive and even more surprising results . Over a period of 18 months, persistence with teriparatide was extraordinarily high, much higher than had ever been seen with other osteoporosis treatments. Now it seemed that dosing was not the only factor leading patients to be nonadherent with their osteoporosis medications. An outstanding review of studies examining adherence revealed that other aspects of medication behaviors such as effectiveness and side effects could well be more important than dosing interval.
72.2.5
Additional bisphosphonates
Two additional bisphosphonates offered alternatives in terms of dosing interval and delivery system. Ibandronate, approved by the FDA in 2003, was initially approved as a daily dose, but its market availability was as a once-monthly alternative . This was the first monthly dosing offered by a bisphosphonate, allowing patients to face the ritual of taking oral bisphosphonates only 12 times a year but still had rigid dosing requirements. Although ibandronate increased bone density in the spine and hip, antifracture efficacy was seen only in the spine .
The monthly option with ibandronate seemed truly promising, and many patients indicated that monthly dosing would be their first choice, and they would be more compliant with it. But when patients understood the lack of efficacy of this medication at the hip compared to the weekly bisphosphonates, their attitudes. For example, the BALTO study found that patients preferred monthly dosing, but patients were not told about ibandronate’s lack of efficacy at nonvertebral sites. When they did have this information, they were far more likely to choose the weekly option . Thus we see again that dosing frequency is not the only variable (or perhaps not even the most important) affecting good adherence.
Ibandronate became available in 2006 as an IV injection given every 3 months . Taking osteoporosis medicine four times a year seems desirable, but because patients could not self-inject ibandronate, they needed to have a health-care provider visit to take the medicine. The last available bisphosphonate was approved in 2007 by the FDA: zoledronic acid. This medication had been approved some years earlier as a monthly infusion for prevention and treatment of bone metastases in cancer patients . The multiple myeloma dose was 4 mL infused over 15 minutes monthly. The osteoporosis dosing was 5 mL infused over 15 minutes once annually . Some thought that zoledronic acid would “ensure adherence” because it was given once yearly and would bypass the GI problems caused by oral bisphosphonates . The single dose of zoledronic acid would be effective for at least a 12-month period . Ironically, 12 months is just about the time when a large proportion of oral bisphosphonate users stop taking their medicine as directed.
72.2.6
Three unique osteoporosis medications
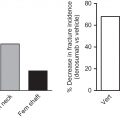
In 2010 the FDA approved denosumab, a fully human monoclonal antibody that has a high specificity for human receptor activator of nuclear factor kappa-B ligand that regulates bone resorption and is unique in the treatment of osteoporosis because it inhibits osteoclast formation and function . Denosumab both reduces fracture risk and increases bone density over time and has a good safety profile . It is not self-injected but must be given by a health-care provider once every 6 months. In a 6-year crossover trial the occurrence of adverse events did not increase with the 6 years use of denosumab . In a series of analyses from the Denosumab Adherence Preference Satisfaction study , subjects showed significantly better adherence to denosumab (injection with 6-month dosing) than with alendronate (weekly oral tablet). This study was an open-label crossover design that required subjects to take both a weekly pill and an every 6 months injection. What researchers could not determine is the reason for better adherence to denosumab. Was it the dosing interval, the means of dosing, or patient attitudes toward these specific medications? Further research may be able to disentangle the relative contributions of each.
An even newer osteoporosis medication was evaluated and approved by the FDA in 2017: abaloparatide. Abaloparatide is a PTH-related protein analog that, like teriparatide, requires daily self-administered subcutaneous injections and also has a 2-year maximum lifetime use recommendation . Like teriparatide, it is an osteoanabolic agent that actually increases bone density in addition to reducing spine and nonspine fractures, including hip fractures. Given its recent approval date, we have no long-term studies of adherence to abaloparatide. However, in a published abstract that reports the results of a real-world observational study, patients indicated high adherence and satisfaction with abaloparatide. Needless to say, further research in this area is essential to determine adherence to abaloparatide, especially in comparison to that of teriparatide .
Most recently, the FDA in 2019 approved a medication for osteoporosis that is a sclerostin inhibitor. This medication is romosozumab that decreased bone resorption and increased bone formation . This is the first osteoporosis medication that has dual action to reduce bone loss and build new bone. According to Khosla , romosozumab may be the closest thing we have to the perfect drug for osteoporosis—at least at the time of its FDA approval. To be able to stop bone loss and increase bone density simultaneously is the hoped-for goal of all osteoporosis treatment .
When initially submitted to the FDA, romosozumab was not approved because of unexpected increased risk of cardiovascular adverse events reported in the Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk of Fracture or ARCH study . According to Saag et al. , during year one of the ARCH trial, serious cardiovascular events were seen more often in the romosozumab group than in the alendronate group. However, other clinical trials of romosozumab showed no such serious adverse events in the cardiovascular arena see, for example, Ref. . Neither did a metaanalysis of six romosozumab data sets show a discrepancy in this area . Thus the FDA gave approval in 2019. Given this recent approval, I could not find any scientific articles examining issues of adherence to romosozumab. However, the dosing schedule (two injections once each month for 12 months) might encourage adherence over daily or weekly therapy. Yet we must remember that dosing frequency by itself is insufficient to improve adherence over time . We must speculate about adherence to romosozumab with an abundance of caution.
72.3
Side effects of osteoporosis medications: their effect on adherence
Whether their actions are simple or complex, all medications have side effects and some level of risk . Some of these side effects are relatively minor and short lived; others may be severe and continue as long as the drug is taken. Certain drugs (as, e.g., chemotherapeutic agents) are well known for their side effects and can be powerful in affecting patients’ quality of life . Dozens of articles report patient refusal of chemotherapy for multiple types and sites of cancer because they cannot tolerate the side effects .
Side effects frequently play a potent role in nonadherence to osteoporosis medications . And during the process of finding effective antiosteoporotic medications that actually are used correctly, both patients and providers must determine whether the efficacy of any medication is worth the possibility of the side effects from that medication. Of course, such a decision requires an understanding of the term “risk” of side effects and a recognizing, if true, that the risk of side effects is less than the risk of a medical consequence of not taking the drug. In fact, one of the biggest challenges to adherence to osteoporosis drugs in general has been the side effect profiles of all these medications .
72.3.1
Antiresorptives: bisphosphonates and other medications
Shortly after the FDA approved alendronate in 1995, reported side effects began to play a substantial role in the prescription of and adherence to these osteoporosis medications. Community users of alendronate reported GI problems shortly after initiating treatment that had not occurred with such frequency in the pivotal trials for alendronate , perhaps because women with existing GI problems (such as peptic ulcers or dyspepsia) were excluded from the trials. Additional support for this hypothesis came from scientists in Denmark and Spain who applied exclusion criteria for the Fracture Intervention Trial to real-life alendronate users . They found that one in two real-life alendronate users would have been excluded from the trials. McHorney et al. found that 67% of nonadherers to oral bisphosphonates in their sample reported side effects as “extremely” or “very important” to the reason for their stopping osteoporosis medicine. Even the adherent patients were concerned about side effects. About 83% said that the absence of side effects were “extremely” or “very important” in their decision to remain on osteoporosis medication.
In addition, Ralston et al. questioned the difference in GI problems between the first two bisphosphonates: alendronate and risedronate. They used data from the Health Improvement Network database in Britain to determine whether 530 patients stabilized on risedronate who were switched to generic alendronate were significantly more likely to experience GI problems afterward. Apparently, some of the generic versions of alendronate used in England had very rapid disintegration profiles that could explain increased numbers of GI incidents . Generic alendronate is, of course, less expensive than the branded version of the drug, something that might improve adherence. However, if the additional GI problems or any other side effects occur with any consistency in generics, nonadherence could increase.
Educating physicians and patients about the importance of following the strict requirements of oral bisphosphonate use did improve the appearance of some adverse events . Many waited to see whether risedronate had a similar safety profile as alendronate. In a retrospective study of administrative claims data on over 6000 patients, Miller et al. found that users of alendronate experienced significantly more GI events than did the risedronate users. This suggests that the safety profile of risedronate might convince patients to select risedronate over alendronate. Later studies of side effects from oral bisphosphonates seem to find few differences between alendronate, risedronate, and ibandronate . Of course, now that these side effects are well known, patients and physicians can make efforts so that side effects do not occur (e.g., follow the dosing information precisely).
Information about one of the most serious side effects from bisphosphonates and denosumab (osteonecrosis of the jaw or ONJ) came from an unexpected source: the dental community. In 2003 Marx wrote a letter to the editor of an oral surgery journal elucidating the problem from his perspective. In it, Marx noted that the Oral Surgery Department of the University of Miami Hospital had seen 36 cases of patients with what he called “avascular necrosis of the jaw.” All of these patients had been on treatment with bisphosphonates (either pamidronate or zoledronic acid). Marx noted that only one patient was receiving a bisphosphonate for osteoporosis treatment; the others were cancer patients who were receiving bisphosphonates because they had cancer. Marx also says that none of the drug evaluations listed osteonecrosis of the jaw (ONJ) as a side effect of the drugs.
The second serious side effect of the bisphosphonates and denosumab was identified in case reports around 2007 and was called atypical femoral fracture or AFF . In the previously cited task force report from ASBMR, a case definition of AFFs was developed; further, no causal link between these osteoporosis medications and AFFs was established. However, the task force was reconvened and provided a second report in order to update both the definition of such fractures as well as clarify major and minor features of these fractures. These fractures appear to be stress fractures or insufficiency fractures and may be caused by extended exposure to bisphosphonates and denosumab. They are extremely rare, with the absolute risk being lower than the risk of hip fracture .
72.3.1.1
Black box warnings
Information about osteoporosis medications and side effects has been widely available. For example, a search of Google for “osteoporosis medications and side effects” resulted in over 8 million hits ( https://www.google.com/search?client=firefox-b-1-d&q=osteoporosis+medications+and+side+effects ). Of course, pursuing individual links will lead to duplications, paid advertisements, and other irrelevancies. But the number of search results indicates that great attention has been paid to these issues. We have noted earlier many of the side effects for osteoporosis drugs and how that might have an impact on patient adherence to said drugs. Yet there is still uncertainty about the validity of these side effect reports and the ultimate safety of any medication aimed at osteoporosis.
Many people have focused on dosing interval and frequency as the most important factors affecting patient adherence. Although these are important, they are far from the only causes of poor adherence. Several outstanding articles have been written describing the role other factors play in affecting how patients make decisions about medications. For example, Lee et al. noted in their review of the published osteoporosis literature from 1970 to 2009 that the decision to take and keep taking an osteoporosis medication rested more on how effective the medicine is (e.g., reducing fractures, increasing bone density) than just on dosing frequency and delivery system. How can patients and physicians learn about differing outcomes of osteoporosis medication? Under ideal conditions, physicians learn these through reading journals and attending conferences. Physicians could then teach patients. Knowledge would travel both ways, however, as patients asked questions that might be answered only through additional physician learning.
A decade or more ago, we could have accomplished that with reasonable ease. People valued physicians’ opinions about health problems, sought advice from them, and generally followed physician recommendations. However, in the 21st century (and perhaps earlier), people seek health advice on diagnosis and treatment from sources other than their physicians . Almost certainly, the information from these sources is neither scientific nor accurate. But it certainly seems imperative to redirect patient searches for health information away from the Internet and social media and back to health-care providers. One case in point is the information about “black box” warnings that have been mandated for several osteoporosis drugs by the FDA.
Black box safety warnings are the highest warning level mandated by the FDA for safety concerns about medications. Raloxifene prescribing instructions had a black box that indicated in 1997 that taking this medication increased the risk of venous thromboembolism and death from stroke . In 2002 the teriparatide label carried a black box that warned of the potential risk of osteosarcoma from the use of this drug . In 2017 the prescribing instructions for abaloparatide had a very similar black box warning as did teriparatide . ( Note : The issue of the transfer of osteosarcoma risk in rats to humans using PTH has received significant attention in the literature and, in the interest of time and space, cannot be covered here. For information, please see Subbiah et al. ). And the newest addition to the pharmaceutical armamentarium for osteoporosis, romosozumab, has a black box warning for potential risk of myocardial infarction, stroke and cardiovascular death . All of the black box warnings outline potentially serious consequences as a possible (though not absolute) outcome of drug use and may frighten away many patients who have need of these osteoporosis drugs .
72.4
A turning point in adherence: serious side effects of osteoporosis medications
Prior to the reports of ONJ and AFFs in the mid-2000s, poor adherence to other osteoporosis medications had already been studied extensively and communicated to professionals and patients alike (see, e.g., Refs. ). Multiple educational programs were pilot tested or examined in randomized clinical trials to identify negative attitudes about the disease and its treatments. The factors that contributed to poor adherence to the oral bisphosphonates, raloxifene, and teriparatide (all FDA approved no later than May 2003) were well known.
An excellent qualitative study of older women’s reasoning behind their nonadherence to osteoporosis medications examined the thoughts and beliefs of a racially/ethnically diverse group of women about osteoporosis . They found that women did not believe that they were at risk of osteoporosis and fractures, that they were concerned about the cost of a therapy they might have to take indefinitely, and that they felt anxiety and fear of the serious side effects from taking a medication they felt they did not need. Another study using conjoint analysis enabled researchers to see how patients diagnosed with osteoporosis made their medication decisions and which of the medication characteristics could be modified . Patients expressed their preferences and weighted them to see which would affect nonadherence most dramatically. There was overwhelming consistency across patients that drug efficacy was the most important medication attributes with safety (aka side effects) the second attribute influencing choice. Cost and convenience seemed less powerful in their impact on medication behavior. Yet nowhere in the commonly read lay literature such as magazines or daily local newspapers is there a clear explanation of how rare either of these side effects are or how great the risk of fracture is if patients elect not to take the medication.
72.4.1
Studies on adherence and intervention trials
Did quarterly or annual dosing of IV bisphosphonates ensure adherence among postmenopausal women? A study done in 2012 addressed this issue. Curtis et al. compared annual IV zoledronic acid to quarterly IV ibandronate with the expressed goal of examining adherence to both but found disappointing results. They ultimately found that adherence was seen in 61% of the zoledronate users and 33%–36% of the ibandronate users. We could draw the conclusion that these findings emphasize that the longer dosing intervals automatically increase adherence. But a numerical increase that does not achieve adherence levels necessary to show meaning fracture reduction is less meaningful (see Ref. ). Instead what this study may have done is to put to bed the notion that dosing interval alone controls patient medication behaviors. While this may have an effect for the short term, it does not appear to have a continuing influence. Although bisphosphonates give women and men multiple choices in dosing interval (from daily to annually) and delivery system (oral pill, infusion, self-injection) to prevent and treat osteoporosis, the extended dosing durations do not result in clinically meaningful adherence rates.
Then multiple intervention studies were initiated to try to identify ways in which patient adherence to osteoporosis medicine in general would improve. Some found improvement in adherence, particularly those that had homogeneous samples (e.g., Refs. ). However, the majority were not successful in increasing adherence to osteoporosis medication . In a review of interventions designed to improve patient adherence to different medications, the authors found that interventions to improve adherence were complicated and effort intensive . They were also expensive. Despite many such interventions, adherence to these medications did not improve significantly.
72.4.2
Side effect information available to patients
Although peer-reviewed journals employ a system of checks and balances that hopefully prevents the publication of unsupported rumors or overexaggerated study findings, the same cannot be said about articles in the lay press or on the Internet. There are, without a doubt, science writers and editors who are honorable and report about osteoporosis and its medications accurately. Two science journalists have recently contributed to major publications read by providers and patients all over the world: Scientific American and The New York Times. In June 2016 Kolata authored an article entitled, “Fearing Drugs’ Rare Side Effects, Millions Take Their Chances With Osteoporosis” . Its purpose was to educate people with or at risk of osteoporosis to be well educated about the costs and benefits of osteoporosis medications. People are saying no to these drugs, as Kolata points out, because of the two exceptionally rare side effects of ONJ and AFF. By rejecting these medications because of fear of rare consequences, patients leave themselves open to worsening osteoporosis. In the end, saying NO to these medications can result in a much great risk of hip or other fractures that can cause major decrements in quality of life, loss of independence, and death.
Patients and providers who read this excellent article and understood it became aware of the threat osteoporosis bring to the public health of this and other nations. But most people read phrases such as “jawbone death” or “red flags raised for osteoporosis meds” as deadly and dreadful; some of them will not even consider taking a medication with such complex and frightening side effects. The most important piece of information communicated to the lay public is that these side effects are extremely rare. These articles are clear, concise, accurate … but are not reaching the people who need to read and understand them.
The impact of these messages about the extremely rare side effects ONJ and AFF is poorly understood in both qualitative and quantitative terms. The most important piece of information about communicated to the lay public is that these side effects are extremely rare. In an excellent article in Scientific American , Wallis states the following: “Then, around 2005, came published reports of two rare but terrifying side effects: osteonecrosis of the jaw and a bizarre breaking of the leg bone called atypical femoral fracture (AFF). A chill descended on the field.” In fact, a chill descended on everyone touched by or at risk of osteoporosis. Yes, it was good to have drugs that could help it but not if the problems they caused were perceived as worse than the disease itself. Clear, concise, accurate … but not reaching the people who needed to read them. And in addition to the examples listed earlier, several websites and stories often contained incorrect postings that catered to those looking for reasons not to take the medications (see, e.g., https://saveourbones.com/top-5-reasons-why-you-should-never-take-osteoporosis-drugs/ or http://drbenkim.com/osteoporosis-drug-dangers ). Well educated and biomedically sophisticated people may know that this is misinformation, those who truly need to understand these issues the most are the least likely to understand that they should not believe everything they read.
Another recent scientific article examined the ways in which knowledge of osteoporosis and its treatment options had improved in the 5 years before publication (2012–16). Yet these authors also point out that, although health-care providers have increased awareness of what osteoporosis is and how to treat it, that information has not been meaningfully communicated to the consumers. The belief that osteoporosis is still only an expected part of aging influences patients to take an apathetic approach toward it. The single exception to this rule occurs in those people who have relatives or friends with osteoporosis and fractures and who have seen for themselves the impairments, pain, and dependence that osteoporosis fosters. The authors applaud the use of fracture liaison services that incorporate multiple services in their efforts to manage osteoporosis .
Researchers from the National Institutes of Health (NIH) studied changes in bisphosphonate use after the media began reporting the frightening side effects of multiple osteoporosis drugs . Using data from Medical Expenditure Panel Survey and the National Inpatient Sample on the use of bisphosphonates from 1996 to 2012, they found that usage dropped significantly during this time and that this decline coincided with the release of safely information on the Internet. During this time, however, neither the US FDA nor the ASBMR recommended any more restrictions on use because of safety concerns.
The Internet is not the only source of inaccurate information about osteoporosis. More traditional media such as newspapers, magazines, and television and radio spots can also contain specious information. For example, National Public Radio (NPR) is usually considered accurate and knowledgeable and relied on by many Americans for credible information about health. But in 2006 NPR presented a show on side effects associated with osteoporosis medications (“All Things Considered,” 2006). In it the station said that doctors are now reporting that, in a small number of people, osteoporosis drugs destroy bones. The introduction of one guest included the following statement: “A few weeks ago doctors discovered that part of his jawbone had died. He had to have emergency surgery to repair a hole in his chin.” The descriptors hole in his chin and jawbone had died are inaccurate and inflammatory. Later in the show, a woman on oral bisphosphonates reported that she had destruction of the jawbone which she described as having terrible sores on the roof of her mouth (emphasis mine). No evidence suggests that diseases of the jawbone also appear on the roof of the mouth, an entirely different part of the oral cavity. Both of these cases became known in or around 2006 at the time when fear of ONJ was picking up traction in the media. They are presented as exaggerated semitruths by a reputable news organization. If radio stations such as NPR are going to promote distorted pictures of the impact of osteoporosis drugs, where can patients turn for more accurate representation? Unfortunately, there may not be such a place at this time, at least not one that commands the respect and support necessary for credibility.
72.5
Can we change pessimism and apathy about osteoporosis and its medications?
We have been inundated with warnings about osteoporosis and its dangerous medications since 1995 when alendronate was introduced as a treatment for this disease. Unfortunately, the good outcomes of osteoporosis treatments have been lost amid the most serious rare side effects of some of the drugs. Changing the patients’ and physicians’ interpretation of this potent negativity will be challenging, especially for those people who have developed a profound distrust of anything associated with osteoporosis . During these two decades, individuals and groups of people have emphasized these negative beliefs toward each new osteoporosis medication and the unique consequences, including esophageal and GI problems, ONJ, and AFF . Given that only a few years earlier medicine had nothing to effectively treat osteoporosis except hormone replacement therapy, successful therapies for this disease should have been welcomed by patients and providers. This joy never happened with osteoporosis medications.
Why have expectations of the negative outcomes of osteoporosis medications continued with every new pharmaceutical pathway? Many women believe that calcium, vitamin D, and exercise are sufficient to ward off the possibility of fractures in the future . Why would they want to add an unknown agent to this mix? But the fact is that nutritional and exercise interventions are simply not enough to prevent bone loss and fractures in people with osteoporosis . Thus the armamentarium of pharmaceuticals and the currents state of osteoporosis knowledge should help us effectively battle this disease. But as the former Surgeon General of the United States, C. Everett Coop, MD, said, “Drugs don’t work in patients who don’t take them!” .
72.6
What now? A fundamental change in public perceptions of osteoporosis and treatment
So many different interventions have been tried—educational, behavioral, and psychosocial—yet nothing shows improvement because the fundamental understanding and awareness of osteoporosis among many people in the United States are flawed. From 1995 until the present, myths have developed around this disease that has no scientific basis or credibility . They have survived, in part, because people in pain or with fractures want to believe in something that fits their perceptions of what osteoporosis is and how it affects individuals.
Some health-care providers believe that their own patients understand fully about osteoporosis and its management, particularly regarding pharmaceutical treatments . They believe that their explanations are clear and complete, and that nothing would cause a patient to misunderstand . This is simply not true. Ample evidence exists that patients of health-care providers from various specialties simply do not leave their visits with a thorough picture of the disease process, a thorough understand of their diagnosis, and a clear treatment plan with which they agree . In the three decades plus since I began to study osteoporosis patients and their quality of life and understanding of the disease, I have repeatedly heard patients complain about the poor communication skills of their providers and their lack of trust in the explanations they have been given. Here are some excerpts from e-mails received between 2016 and 2019 from women with osteoporosis who should understand the disease they have had for decades. All women have given permission for this use with the stipulation of remaining anonymous.
- •
I worked for 25 years, retired at 62, and was frightened re osteoporosis. … going off into retirement, after breaking fingers, ribs, elbow and shoulder, I was fearful of broken back/hip/whatever. I had lost half of my bone mass. I don’t know how low my T-score is now, as I do not go for bone scans any more at 83.
- •
I took Actonel and Fosamax, and now we know the bad side effects. No longer in use. I did not see bone growth results. Also my esophagus suffered. … and then stopped ALL osteoporosis meds. Now they say if you have GERD issues, stop the meds immediately that cause it. I did take one infusion med. No bone mass difference.
- •
I go to dentist every 3 months (due to Actonel/Fosamax and their causing the possibility of jaw cancer if a tooth is extracted). Frightening. I have x-rays as needed.
- •
I do not take osteoporosis meds because I do not trust the FDA. Nor the drug manufacturers, the drug lobbyists or the drug reps, and my own horrific experience.
These patients provide clear evidence that they do not understand about osteoporosis correctly and that they have bought into some of the myths about this disease and its medications.
72.6.1
How can we change patient perceptions?
Instead of developing more clinically based interventions that do not seem to work, we must turn our efforts in a different direction. We need to effect a perceptual change on the part of the general public, the medical community, and osteoporosis patients in particular in medication decision-making . Fundamentally, we must erase the negative views of osteoporosis and its treatments that began to emerge in the mid-to-late 1990s and replace them with convincing and honest information that can be spread across three groups: people with osteoporosis, health-care professionals, and the rest of American society. Prior to 1995, osteoporosis was accepted as a part of the normal aging process that could not be treated or prevented. Current lay information about osteoporosis (as sampled previously in the bullets) suggests that, 25 years later, we have made little progress overall in teaching the public about the real issues of the disease: loss of bone density, fractures, disability, deformity, and death . The emergence of false perceptions about the disease, medications, and consequences can be laid at the feet of the media and others using scare tactics to increase readership . But if we can help patients ask their physicians the right questions and to understand the concept of risk (i.e., risk is associated both with the medicine and with not taking the medicine), the health-care professionals will modify their perceptions of osteoporosis and its treatments as well. Few behavioral and social scientists have adapted any of the efforts to cognitively reframe attitudes about osteoporosis. Perhaps the perceptions of those who study the psychosocial impact of cancer and rheumatoid arthritis and diabetes do not see osteoporosis as causing the same level of distress or suffering. The concept of cognitive reframing was first introduced in the literature after Beck began to use it with patients with depression. His much-cited 1970 article makes the following critical statement: “Systematic study of self-reports suggests that an individual’s belief systems, expectancies, and assumptions exert a strong influence on his state of well-being, as well as on his directly observable behavior. (p. 184)” .
Important social science research has shown that patient perceptions of osteoporosis and fracture are neither thorough nor correct. For example, Sale et al. studied patient perceptions of fragility fractures and whether they connected such fractures with having their own osteoporotic fractures. They found that patients experienced their own fractures as traumatic both physically and emotionally. To the patients, “fragility” did not correspond to the fracture experience they had. For many older adults, “fragility” was a misnomer; for them, there was a disconnect between their own experiences and the words used by providers to describe the disease they had. The effort and time necessary for cognitive retraining are substantial. But perhaps we have reached a point where doing less is simply a waste of resources and of time.
72.7
Conclusion
We are at the end of the second decade of the new millennium and have many more medications approved for treatment of osteoporosis. There are different types of medicines, different delivery systems, and different dosing regimens as well as different outcomes. These medicines work through different pathways in the human body to maximize bone health. Yet even at this time, perhaps the most significant problem we still have in osteoporosis management is our failure to enable patients to comply and adhere to any of the osteoporosis medications. We have not yet reached patients and physicians in a meaningful way to help them truly understand what these medications do and how their use can decrease fracture risk and improve quality of life. For the mission that began in the mid-1990s—that is, the efforts to have people with osteoporosis be treated for their disease effectively and safely—we see few positive trends in medication behaviors or in the understanding and attitudes toward osteoporosis. At best, we can report no change over the last several decades. At worst, we could say that our attempts to improve compliance and adherence to antifracture medicines have taken steps backward. For the people who suffer from osteoporosis and the fractures associated with this disease, our lack of progress is regrettable. For the people who treat osteoporotic patients, conduct research on bone in multiple arenas, and those who try to translate research findings into real-world changes, our lack of progress is disheartening and embarrassing.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree