Fig. 34.1
Affected range of motion of right shoulder after full axillary clearance
Factors associated with an increased risk of postoperative complications include:
Age
Obesity
Smoking
Excessive use of alcohol or recreational drugs
Diabetes
Chronic renal failure or chronic obstructive pulmonary disease
Atherosclerosis and cardio-vascular disease
Autoimmune and connective tissue disorders
Preoperative chemotherapy
History of irradiation to the chest wall
Previous surgical procedures on the breast
Breast surgery complications may vary in severity, from severe to mild. Life-threatening complications are very rare. Seroma, infections requiring antibiotic treatment only, partial flap or nipple necrosis, limited bleeding and delayed wound healing are classified as mild. Severe complications are those requiring hospitalisation, repeat surgery, blood transfusion, total or extensive flap or nipple necrosis and implant removal.
As with all surgical complications, classification of severity is helpful for undertaking high-quality audits and research. A number of such systems are in use. The most popular is the Clavien-Dindo system, which is very simple to use and modifiable for any complication or disease site (◘ Table 34.1) [2]. More complex is the Common Terminology Criteria for Adverse Events (CTCAE, V 4.0) system which is much more research specific but requires reference to specific criteria for each complication and is not as easy to use [3].
Table 34.1
The Clavien-Dindo simplified classification of surgical complications
Grade I | Any deviation from the normal postoperative course that can be treated with simple medications (antiemetics, antipyretics, analgesics, electrolytes, physiotherapy) |
Grade II | Requiring pharmacological treatment other than the above and/or blood transfusion |
Grade III | Requiring surgical intervention |
Grade IV | Life-threatening complications with organ dysfunction and/or need for ICU management |
Grade V | Death |
34.2 Bleeding and Haematoma
The breast has a rich network of small arteries. Usually these arteries are branches of the internal mammary artery, the intercostal arteries, the lateral thoracic artery and the thoracoacromial artery. Some of these vessels can be missed during the stage of haemostasis as they tend to retract inside the bulk of the pectoralis major muscle and may cause significant postoperative bleeding. Furthermore, the tributaries of the axillary vein require special attention during axillary node clearance, as failure to identify and ligate these may result in significant bleeding as well as further injury to the vein during attempts to achieve haemostasis. Finally, in cases of preoperative chemotherapy, the involved and previously matted axillary lymph nodes tend to regress and can mislead the surgeon into believing that planes along vessels have been maintained; thus in the effort to dissect them, it is possible to cause injury to the axillary vein or other large vessels.
The frequency of postoperative bleeding is difficult to estimate, as small haematomas of minimal clinical significance are common. In contrast, significant haemorrhage and large, expanding haematomas which require blood transfusion and/or surgical intervention are unusual. The incidence of haematoma after breast surgery without reconstruction is reported to be between 2% and 10%. It is possible that more complex procedures that involve reconstruction or intervention to the contralateral breast have a higher risk of bleeding [4, 5]. The early use of a good quality brassiere, which applies mild but steady pressure to the breast, may reduce this risk in cases of conservation. Areas that can also bleed are the donor areas of the back (latissimus dorsi (LD)) or the lower abdomen (transverse rectus abdominis myocutaneous (TRAM) and deep inferior epigastric flaps (DIEP)) when reconstruction with autologous tissues has been carried out [5, 6]. If a submuscular implant is used, the vessels between the chest wall and pectoralis major muscle also need attention.
Anticoagulant therapies are associated with an increased risk of postoperative haemorrhage and must be managed appropriately. Non-steroidal anti-inflammatory drugs (NSAIDs) are associated with a slightly increased risk of postoperative bleeding, and it is advisable (but not essential) that they are discontinued a week before complex procedures. The risk with most NSAIDs is not great; however more recent agents inhibiting platelet function such as clopidogrel do carry a greater risk, and discontinuation is strongly advised before routine surgery. Warfarin must likewise be discontinued prior to surgery to allow the internationally normalise ratio (INR) to fall to 1.5 or less. Depending on the indication for warfarin, a bridging anticoagulant protocol to cover the perioperative period may be required. Low molecular weight heparin (LMWH) for the prevention or treatment of deep vein thrombosis (DVT) or atrial fibrillation complications may increase the risk of bleeding, especially if given at therapeutic rather than prophylactic doses and again should be stopped before surgery. It is worth mentioning that, although considerable blood loss into the drain may occur, drains often fail to drain haemorrhage due to clot formation in the surgical cavity or within their lumen. In these cases extensive bruising and oedema of the breast or chest wall are usually visible [7, 8].
Reoperation is indicated in cases of large, expanding haematomas – especially when painful or at risk of causing wound dehiscence – or where hypotensive shock is present (rare) or blood transfusion is required. Often there is no visible bleeding vessel and haematoma evacuation is adequate; however, it is wise to ensure normotensive anaesthesia before closure in case the bleeding vessel is pressure sensitive. Meticulous washing and haemostasis facilitate healing, improve cosmesis, reduce postoperative pain and prevent delay of adjuvant treatment. The decision to reoperate to control bleeding must be taken earlier if reconstruction has taken place, as haematomas can affect the blood supply to vascular pedicles or increase the risk of infection.
34.3 Surgical Infections
These are usually caused by skin flora, most commonly Staphylococcus. The incidence varies among studies from 10% to 20% [9, 10]. Autologous or silicone implant-based reconstructions do not seem to be associated with higher rates of infection, but the consequences are considerably more significant. The use of acellular dermal matrices for reconstruction is however associated with a higher infection risk [11].
Risk factors for infections include age, obesity, diabetes, smoking and recent chemotherapy. Hypertension, ASA score 3 or 4, a history of previous breast surgery, haematoma and lengthy or bilateral procedures have also been reported to increase the risk [12–14].
Radiotherapy given after silicone implant-based reconstruction increases the risk of infection (as well as the risk of other local complications such as skin necrosis, wound dehiscence and capsule contracture) significantly and independently of other risk factors (◘ Fig. 34.2) [15–18].
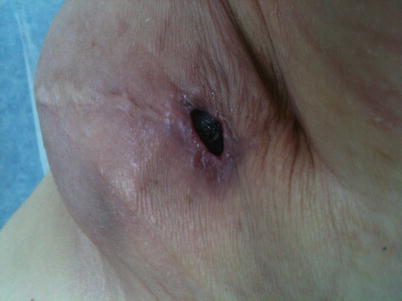

Fig. 34.2
Inflammation and wound break down after mastectomy with immediate silicon based reconstruction followed by radiotherapy
Surgical infections may be prevented with antibiotic prophylaxis, simple penicillins or cephalosporins are often used but gentamycin may also be added when reconstruction is taking place. Some surgeons prefer to administer antibiotic prophylaxis even in the absence of risk factors, by giving a single dose intraoperatively or covering for 24 h. Notably, antibiotics have not been proven to be more effective if given for more than 2 days [14, 19].
Treatment of surgical infections must be given in a timely manner to prevent delay to adjuvant therapies. Simple cellulitis is treated with antibiotics against skin flora on an outpatient basis. Infective collections should be drained surgically by inserting a drain in the cavity through which lavage can be done. Small abscesses after conservation surgery may be treated with needle aspirations that may have to be repeated several times; in this way, reoperation is avoided but recovery may be slightly delayed.
Infections related to silicone implants or tissue expanders are much more difficult to treat. If the implant comes into contact with infected material, its removal may be the only option (◘ Fig. 34.3 ). Needless to say that any other artificial materials used for the reconstruction may have to be removed too (ADMs and meshes). Meticulous wash out and use of drains for a few days are also mandatory. Rates of implant loss due to infection vary in published literature between 0% and 2% [20, 21]. Repeated attempts for reconstruction should then be delayed until all infection has been effectively treated and scarring has matured (a few months). In such cases, reimplantation is associated with a high rate of further infection of up to 50%, and a flap-based, fully autologous technique may be safer. Recently there have been reports of implant-based reconstruction salvage after cavity wash out, implant exchange and antibiotic use. Implant salvage rates can exceed 50% in these series and depends mainly on the severity of the infection [22–24].
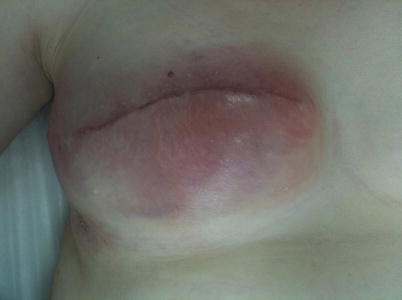

Fig. 34.3
Bacterial infection after delayed breast reconstruction presenting with erythema and local sensitivity. The implant was removed
34.4 Neuropathic Pain and Muscle Weakness Due to Nerve Damage
A percentage of patients who undergo breast surgery will develop persistent chronic postoperative pain. This may occur after any surgical procedure to varying degrees, but breast surgery seems to be one of the most common causes with an incidence of up to 60%. It may affect the breast, the chest wall and the arm, especially its upper inner surface. The pain can feel like burning, cold or paraesthesia and may be associated with a trigger point, but there may be no other symptoms or signs indicative of a problem. The pain can occasionally be severe and necessitate treatment with serotonin reuptake inhibitors such as pregabalin or gabapentin. Common analgesics are not usually very effective. Some improvement may be gained by low-dose antidepressant therapy such as amitryptyline. In cases where there is a definite trigger point, focal injection with local anaesthetic and steroids may help. Risk factors for neuropathic pain are young age, breast conservation, radiotherapy, chemotherapy, depression and axillary surgery, especially full axillary clearance [25, 26].
Dysaesthesia and/or paraesthesia can also affect the surgical area due to damage to sensory nerves. Specifically, injury to the intercostal brachial nerves, which run across the axillary cavity from medial to lateral, will result in persistent paraesthesia along the upper inner surface of the arm. Efforts to save these nerves during surgery are often ineffective or even cause worse symptoms, probably due to their inevitable injury or devascularisation [27].
Paraesthesia of the nipple is common, especially after procedures which involve significant undermining of the nipple-areola complex. The symptom tends to improve with time, but impaired sensitivity is not uncommon and patients should be warned in advance. Thus, nipple numbness persists in 35% of patients 2 years after an inverted-T breast reduction and in 21.5% after inferior pedicle procedures [28, 29]. For patients receiving surgical treatment for recurrent periareolar infections, permanent loss of nipple sensation is the norm.
The cords of the brachial plexus lie cephalad to the axillary vein, relatively protected from direct surgical trauma during axillary procedures. Injury, however, is possible, usually due to prolonged abduction of the ipsilateral arm of more than 90° intraoperatively. When the cords or branches of the plexus are injured, symptoms may include muscle weakness or total paralysis. Because a complete division of a nerve is highly unlikely – and neurapraxia much more likely than severance – symptoms usually subside with time. Early physiotherapy, steroids and vitamin B complex supplements are included in the treatment. When neuropathic pain is also present, it can be treated with antiepileptics, antidepressants or common opioid or non-opioid analgesics [30–34]. Risk factors for neurological symptoms are preoperative chemotherapy – which is frequently neurotoxic, obesity and lengthy surgical procedures. It is rare for early breast cancer-affected axillary nodes to directly invade the nerves or vessels requiring their sacrifice except in cases of very obviously advanced disease where neoadjuvant therapies are preferable to surgery in the first instance.
Division of the thoracodorsal bundle must be avoided unless directly invaded by the tumour, a condition which is rarely seen. Deprivation of the LD muscle from its nerve stimuli results in muscle weakness and difficulty in medial rotation, adduction, extension and hyper extension of the arm. Sacrifice of the arterial supply to the LD precludes breast reconstruction with this muscle. It is worth mentioning, however, that because the movements of the shoulder are the result of groups of muscles working in combination, LD muscle weakness or even its complete absence from its natural position has minimal impact on most daily activities with a few exceptions; this explains why the use of this muscle in breast reconstruction carries an almost negligible risk of significant disability.
Similarly, sacrifice of the medial and lateral pectoral nerves, which innervate the pectoralis muscles, results in muscle weakness and atrophy. These nerves can be injured during insertion of submuscular implants or an intermuscular approach to the apical axillary nodes.
The most significant nerve to injure during axillary procedures is undoubtedly the long thoracic nerve (of Bell). This nerve arises from the anterior rami of the fifth, sixth and seventh cervical nerves; then it descends behind the brachial plexus and the axillary vessels, along the lateral surface of the serratus anterior. It must be identified and preserved during axillary node clearance. It can be usually found on the outer surface of the serratus anterior, relatively close to the axillary vein. When all the axillary tissue is detached from the lateral thoracic wall, the nerve is pulled towards the specimen side, and the surgeon can identify it as a cordlike structure running in a cranio-caudal direction. Another area where it can be damaged is higher up, where it crosses the axillary vein, during the dissection of levels 2 and 3 of the axillary lymphatic tissue.
The most typical symptom of long thoracic nerve damage is projection of the scapula backwards, either spontaneously or when pressure is applied with the ipsilateral arm on a hard surface (winged scapula) (◘ Fig. 34.4). Pain, weakness – especially when the arm is flexed – and medial displacement of the scapula are common too. It seems that this complication is more frequent than generally believed, reaching 10–30% in some reports. With time, however, it tends to resolve, as the nerve injury is often only temporary (neurapraxia) and rectifies itself without treatment. Eventually, less than 3% of patients who undergo full axillary clearance will end up with permanent symptoms. Generally speaking, a «winged scapula» is treated with physiotherapy and orthopaedic braces if necessary. If symptoms persist for more than a few months, surgical treatment may be indicated and includes muscle or tendon transfers or fixation of the scapula to the rib cage [35–37].
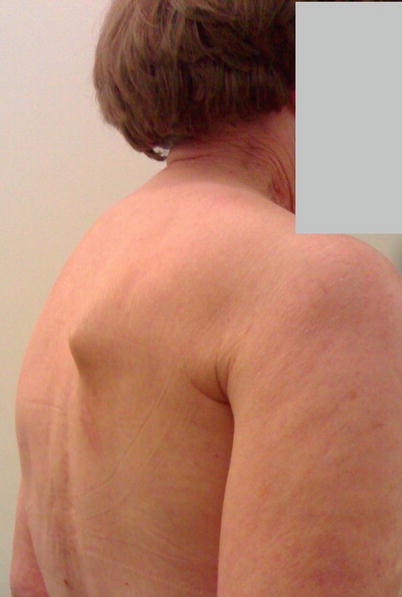

Fig. 34.4
Winged scapula after full axillary clearance
A different group of nerve injuries may result at the donor sites of autologous tissue reconstruction. The most common relevant complication is weakness of the anterior abdominal wall after DIEP or TRAM flap reconstructions due to division of the nerves entering the rectus abdominis muscle through its lateral edge. Thus, even if the muscle remains in situ, the bulging of its most inferior part or even the development of a true hernia is not uncommon.
34.5 Seroma
Seroma is the collection of serous fluid within the surgical cavity after excision of part or all of the breast or axillary tissue. Usually these are the spaces between the muscular floor and the skin after mastectomy or conservation surgery and the axillary cavity after full nodal clearance. The pathophysiology of seroma formation is not entirely clear but is a very common condition occurring in up to 80% of cases [38]. It is often considered not a true complication but an inevitable consequence of surgery. Its clinical significance is usually minor, but it may cause concerns to the patient and require repeated drainage.
The symptoms of seroma formation are subtle. There may be discomfort or a vague sensation of heaviness. Occasionally, serous collections may leak through the skin (usually through the suture line), causing anxiety to the patient. Rarely seromas can cause significant pain, delayed healing, wound dehiscence, infection and compromise of the range of movement of the shoulder. Seromas increase the risk of infections during adjuvant chemotherapy and might cause difficulty to the planning of radiotherapy treatment. Seromas associated with ADM reconstructions may reduce the speed of vascularisation of the ADM and so increase the risk of infection. For this reason prolonged drainage is advised [39–42].
It has been suggested that serous collections at the surgical site are related to the division of lymphatic vessels and leakage of lymphatic fluid. However, it is known that the composition of seroma changes after a few days, eventually becoming an exudate. Diathermy causes thermal injury to the surrounding tissue, which responds with fluid production [1].
Due to the high frequency of this complication, there have been several suggestions on how to prevent it. Ultrasonic scissors, pressure dressings, tacking with sutures, avoidance of electrocautery, application of agents to the surgical cavity which are supposed to facilitate the adherence of its walls to each other and eliminate dead space and the restriction of arm use in the first few days have all been suggested, but trials have not been convincing, usually presenting mixed results (◘ Table 34.2) [43–86]. The use of drains may reduce the size of seroma, and surgeons tend to keep them in situ from 1 day to 1 week or even longer especially when reconstruction has taken place [87–89]. The treatment of seroma is simple and consists of transcutaneous needle aspiration which may have to be repeated several times. Sometimes the seroma persists for several months and a reoperation is needed.
Table 34.2
Methods used to reduce postoperative seroma
Technique investigated | Reference | Comment |
|---|---|---|
Harmonic energy | Iovino et al. [43] He et al. [44] Böhm et al. [45] Kozomara et al. [46] Galatius et al. [47] Kontos et al. [48] Yilmaz et al. [49] Deo et al. [50] Khan et al. [51] Rohaizak et al. [52] Ribeiro et al. [53] | In some published trials, the use of harmonic energy has been shown to reduce postoperative seroma and/or duration of drainage after breast cancer surgery, especially when compared to monopolar electrocautery. However there is a large number of publications supporting that the use of harmonic scalpel is not beneficial and also more expensive. Meta-analyses results are also mixed [54–56]. Even in publications with results favourable for harmonic scalpel, drains were invariably used, and postoperative aspirations of serous collections were probably less but still necessary |
Fibrin glue | Sanders et al. [57] Kulber et al. [58] Butler CE [59] Jain et al. [60] Cipolla et al. [61] Gilly et al. [62] Moore et al. [63] Vaxman et al. [64] Mustonen et al. [65] Ulusoy et al. [66] | Despite the evidence from animal models that fibrin sealant reduces postoperative seroma, clinical applications gave variable results. A relevant meta-analysis concludes that there is no benefit from its use [67] |
Tacking sutures | Purushotham et al. [68] Purushotham et al. [69] Classe et al. [70] Schuijtvlot et al. [71] Ouldamer et al. [72] Ten Wolde et al. [73] Ouldamer et al. [72] van Bastelaar et al. [74] Sakkary MA [75] | There have been numerable reports on techniques of mechanical suturing of the mastectomy or/and axillary lymphadenectomy flaps to the underlying muscular structures. The mechanism is to reduce dead space, accelerate healing and possibly avoid using drains. Different results have been reported although there must be at least some degree of benefit from the technique. A relevant literature review [76] also suggests that closure of the dead space by flap fixation with sutures reduces seroma formation and the number of aspiration |
Pressure dressing | Chen et al. [77] O’Hea et al. [78] Kontos et al. [79] | External pressure to the area of mastectomy with special garments or dressings is based on the idea of elimination of the dead space and keeping the mastectomy flaps in constant contact with the chest wall to facilitate adherence. Again only one a trial reports good results, and even then many patients underwent extra clinic visits and repeated aspirations |
Restriction of arm use | Chen et al. [80] Abe et al. [81] Jansen et al. [82] Petrek et al. [83] Knight et al. [84] Schutz et al. [85] | The restriction of the use of the ipsilateral arm after breast cancer surgery aims to fast local healing and elimination of the surgical cavity. The restriction has been applied for a variable period of time, sometimes until drain removal. The published results, however, are very diverse, and a relevant review concluded that although there may be a degree of benefit with regard to seroma formation, safe conclusions regarding the shoulder long-term function cannot be made [86] |
Risk factors for seroma formation are age, obesity, use of electrocautery, size of the tumour, size of the breast, full axillary clearance and number of lymph nodes removed [90–95].
Seroma formation after silicone implant-based reconstruction needs special attention. Generally speaking, drains tend to be left in place for longer and are removed when output is less than 20–40 mls per day. This gives time for some healing to take place as well as stabilisation of the surgical cavity. The presence of a large seroma may cause an anatomically shaped implant to rotate or shift position if permitted to occur. However prolonged use of drains may increase the risk of infections. In the case of seroma formation around a silicone implant or a tissue expander, aspiration and drainage may be done under aseptic ultrasound guidance if it becomes tense or uncomfortable.
34.6 Cording and Range of Movement of the Shoulder
It is well established that surgical procedures involving axillary node clearance or, to a lesser degree, sentinel node biopsy may affect the range of motion of the shoulder. This complication was underestimated in the past, and the advice given to patients used to be that the movements of the ipsilateral arm should be kept to a minimum for days or weeks in order to minimise the risk of seroma formation. Today, patients start abducting and flexing the arm early in the postoperative period, and with the exception of a few elderly patients this complication is rare. Moreover, the widespread use of sentinel node biopsy which reduces axillary scaring also contributes towards the better results. Physiotherapy before and after surgery is helpful and often routinely offered in many units to reduce this complication [96, 97].
The range of the motion of the shoulder can be impaired in up to 25% of patients following axillary node clearance (◘ Fig. 34.1). However, with time and physiotherapy rates can considerably improve [98, 99]. It is preferable to provide pre-emptive physiotherapy and exercise advice than to treat the problem after it is established. It is obvious that nerve damage, especially to the long thoracic nerve of Bell, significant neuropathic pain and radiotherapy to the axilla worsen the problem. Finally, it is worth noting that severe impairment of the range of motion of the shoulder complicates adjuvant radiotherapy setup and planning.
A specific type of scarring in the axilla is the formation of elongated fibrous cords which may occur in 5–10% of cases. These can extend outside the axillary area and reach the elbow or even the forearm. They cause significant deterioration of the range of the movements of the shoulder and may take weeks or months to resolve. Physiotherapy is of paramount importance to achieve their resolution.
34.7 Delayed Wound Healing and Wound Dehiscence
Delayed healing of a surgical incision is not uncommon in breast surgery. Its severity can vary from simple delay of the healing process to actual failure of the suture line and true dehiscence. Tissue necrosis is not a prerequisite; loss of the ability of tissues to heal in a timely manner is enough to cause this complication.
It is difficult to estimate the actual frequency of delayed healing, as it has not been agreed upon what constitutes a delay and because often a simple and short delay has no further implications. In cases when there is necrosis of the underlying tissues (e.g. fat necrosis), there is an increased risk of infection. Often, additional interrupted sutures and thorough local asepsis buy time for the healing process to be completed. Good surgical technique and avoidance of ischaemic tissue flaps prevent this complication. A history of radiotherapy or surgical procedures in the area, recent chemotherapy, smoking, poorly controlled diabetes and older age seem to be of the most common risk factors. Specifically for smoking, this should be stopped at least a month before surgery in order to minimise its adverse effects on the tissues locally [6, 100, 101]. This is of critical importance for complex flap-based procedures such as mastopexy, autologous free or pedicled flaps and implant-based procedures where smoking increases the risk of complications several fold in most series [102, 103].
Extensive tissue manipulation, which often takes place in oncoplastic and reconstructive surgery, can result in damage to the blood supply of the flaps. Good surgical technique, adequate training and a thorough understanding of the blood supply of flaps are necessary in order to avoid these complications, although anatomic variants mean that they are occasionally inevitable.
If silicone implant-based reconstruction has taken place, the prevention of wound complications is of paramount importance. The underlying pectoralis major muscle often protects the implant from exposure in case of wound dehiscence; however, if the incision is placed at the inferior aspect of the reconstructed breast, it is possible that a wound break down will leave the implant or the ADM exposed and vulnerable to infections. Practically speaking, it is difficult to avoid implant and ADM removal if they become visible though a dehisced suture line, as they are usually already infected. Certain incision types are more prone to a risk of wound breakdown and should be used with caution in high-risk cases. High-risk mastectomy incisions for skin or nipple necrosis include the wise pattern or mastopexy incisions and periareolar incisions. Indicatively, after nipple sparing mastectomy and immediate reconstruction, nipple necrosis has been reported at 8–10% for periareolar/circumareolar and mastopexy incisions, 18% for inframammary and as high as 82% for transareolar incisions [104].
34.8 Lymphoedema of the Breast and Associated Infections
After breast conserving surgery – especially if it involves axillary node clearance and radiotherapy – it is possible for the remaining breast to develop lymphoedema presenting with the typical appearance of peau d’orange. The breast has an oedematous and often erythematous appearance, giving the impression of a bacterial infection. Skin oedema and peau d’orange are typical, and often the condition recurs repeatedly. The appearance of the breast can also resemble inflammatory cancer recurrence. For this reason, mammography, MRI and biopsy of the most severely affected area of the skin or parenchyma may be appropriate to rule this out [105]. Microbiology samples are usually negative. Lymphoedema of the breast is reported in up to 5% of conservation cases. Almost always it occurs within the first year postoperatively, and it can persist for more than 6 months or even be permanent. It seems that damage to the lymphatic system as a result of excisions in the upper outer quadrant, axillary node clearance, radiotherapy and postoperative haematomas can cause this condition [106]. It is also more common in patients with large breasts, with a large volume of resected tissue, with arm lymphoedema and with more than one breast biopsy or seroma aspiration. Resolution may require physiotherapy and lymphatic massage techniques to encourage alternate lymphatic drainage channels to open up [107]. The use of a well-fitted brassiere which supports and gently compresses the breast tissue can drain the oedema from the breast and potentially prevent infection. If infection is present, antibiotics can be used. Extremely rarely and only as the very last resort, mastectomy can be discussed when infectious episodes are frequent and severe and antibiotic therapy is practically continuous.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







