Complement
Learning Objectives
• Recognize the biologic functions of the complement cascade
• Identify the components of the classic complement pathway
• Discuss the roles of anaphylatoxin in an immune response
• Recognize the biologic function of C2b
• Name the complement components in classic pathway C3 convertase
• Relate complement activation to microbial insolubility, osponization, and phagocytosis
• Recognize the three different anaphylatoxins generated in complement pathways
• Identify the components and function of the membrane attack complex (MAC)
• Differentiate between the classic complement pathway and the alternative complement pathway
• Describe the unique structure and function of C9
• Identify the molecules involved in the control of the classic recognition complex
• Analyze the role of C3 in the activation of the alternative complement pathway
• Name the components of the C3 convertase in the alternative complement pathway
• Recognize the proteins that control the activation of the alternative C3 convertase
• List the seven molecules expressed on mammalian cell membranes that inhibit complement activation
• Identify the role of complement in B cell activation
• Identify the role of complement in the generation of memory cells
• Describe the immunologic defect in hereditary angioneurotic edema (HANE)
• Design a successful treatment regimen for HANE
• Describe the immunologic defects in paroxysmal nocturnal hemoglobulinuria (PNH)
Key Terms
Activation complex
Anaphylatoxin
Antigen recognition complex
Alternative complement pathway
C1 esterase inhibitor (C1INH)
C3 convertase
C4 binding protein (C4BP)
C5 convertase
CD59
Classic complement pathway
Complement receptor I
Decay accelerating factor (DAF)
Factor H
Factor I
Hereditary angioneurotic edema
Homologous restriction factor (HRF)
Membrane activation complex
Membrane cofactor protein (MCP)
Metastable C3
Paroxysmal nocturnal hemoglobulinuria
Properdin
S protein (vitronectin)
Introduction
Complement is a family of more than 20 plasma proteins that includes enzymes, proenzymes, enzyme inhibitors, and glycoproteins. Components interact in cascades and assist in the resolution of a microbial infection. For example, fragments indirectly render the microbe insoluble and draw phagocytic cells to the area of infection. Other fragments coat the microbe and act as opsonins, which promote phagocytosis. In a third function, complement components create lesions in the microbial cell wall that result in osmotic lysis. Complement fragments also provide the second signal necessary for B cell activation.
Three different complement cascades have been described—classical, alternative, and the mannose-binding lectin (MBL) pathways (see Chapter 2). Only the classic pathway requires an antibody for complement activation. In the MBL and alternative pathways, complement components are directly activated by highly conserved microbial antigens.
Classic Complement Pathway
The classic pathway was the first described using sheep red blood cells (SRBCs) coated with anti-SRBC immunoglobulin M (IgM). Over a number of years, nine complement proteins were found to be involved in the cascade. These proteins interact in the following sequence: Ab:C142356789. On the basis of function, the classic cascade is subdivided into recognition, activation, and membrane attack complexes.
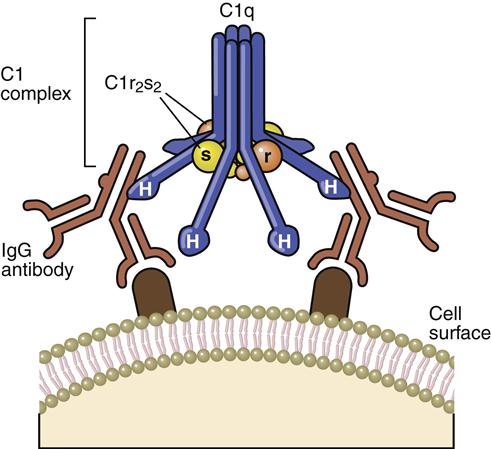
Recognition Complex
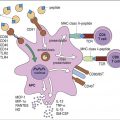
The recognition complex consists of antibody (Ab) and C1. The C1 component is a complex of three proteins (C1q, r, and s). C1q is composed of polypeptide chains with a triple helix “collagen-like” amino terminus and a globular structure at the carboxyl terminus (Figure 11-1). C1r and C1s are inactive serine esterases.
When the antibody binds to the antigen, a conformational change occurs in the hinge region, which exposes complement receptors. Although both IgG and IgM can activate the complement cascade, IgM is more efficient because of the proximity of multiple hinge regions. In the initial step of complement activation, C1q bridges two hinge regions. In the next step, C1r and C1s associate to form a tetrameric complex (two molecules of each protein), which binds to C1q. In the presence of calcium, C1r activates the serine esterase activity of C1s.
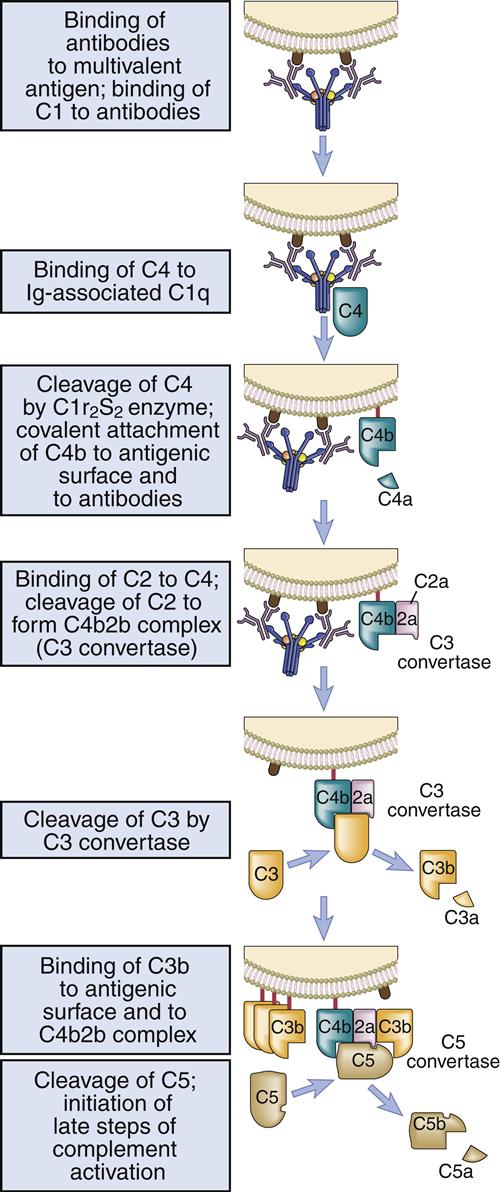
Activation Complex
Activated C1s cleaves multiple C4 and C2 molecules. C4 is a large 210-kDal trimeric polypeptide, which contains an unusual thioester group. C4 is cleaved into C42a and C4b. C4a is a soluble protein that attracts phagocytic cells into the area where complement is being fixed. The C4b fragment is coupled to the microbial cell. Cleavage of C2 results in the production of C2a and C2b. The larger C2a (75-kDal) fragment remains in contact with C4b to create an AbC4b2a complex on the cell surface. Plasmin cleavage of the smaller, soluble C2b fragment creates a kinin, which causes leakage of fluid from vessels and tissue edema.
C3 Convertase
The C4b2a complex, or C3 convertase, catalyzes the cleavage of C3 into C3a and C3b. This is the most important step in the complement cascade and occurs in the classic, alternative, and MBL pathways. C3b is a highly unstable molecule that has a unique thioester that allows covalent binding to a microbial cell. Some C3b fragments bind to the target cell, where they act as opsonins. In the classic pathway, most C3b is found in an AbC4bC2aC3b complex on the cell surface, which is called the C5 convertase.
The soluble C3a fragment acts as an anaphylatoxin. By definition, an anaphylatoxin is a molecule that induces the movement of eosinophils and phagocytic cells (chemotaxis) toward increasing concentrations of C3a (Figure 11-2). Anaphylatoxins also release histamine and heparin from basophils and mast cells. Histamine and heparin cause vasoconstriction and increased vascular permeability, which reduce the solubility of microbes or antigen–antibody complexes.
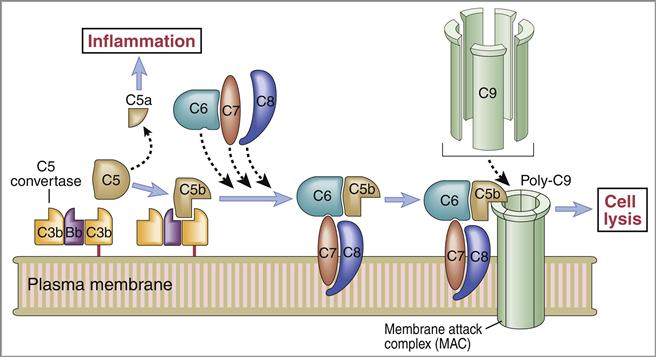
Membrane Attack Complex
The C5 convertase cleaves C5 into C5a and C5b fragments. The C5b binds to the cell surface and serves as a platform for the membrane attack complex (MAC), which consists of C5bC6789 (Figure 11-3). C5a is the most potent anaphylatoxin (100–1000 times more potent than C3a) in the complement cascade.
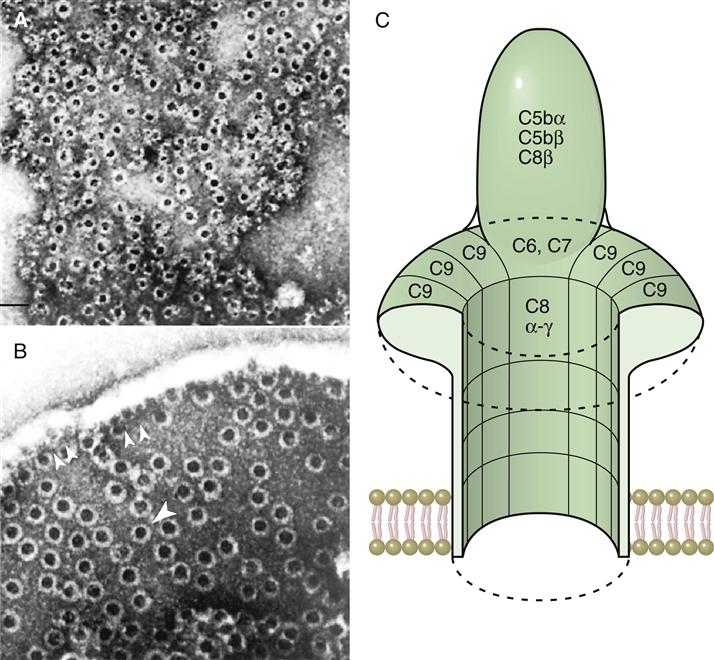
Binding of the C6C7 component creates a trimolecular, lipophilic complex, which is inserted into cell membranes. C8 also is inserted into the microbial cell wall or membrane, further increasing the stability of the C5b678 complex (Figure 11-3). Polymerized C9 is inserted into the cell membrane and creates pores in the cell membrane that cause osmotic lysis (Figure 11-4).