EARLY EVENTS AND CRITICAL PATHWAYS IN COLORECTAL TUMORIGENESIS HIGHLIGHTED BY INHERITED SYNDROMES OF INCREASED CANCER RISK
Two uncommon but highly penetrant inherited syndromes, familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC), together account for about 5% of all CRC cases. Other rare syndromes, familial juvenile polyposis (FJP), Peutz-Jeghers syndrome (PJS), and Cowden disease, each occurring in fewer than 1 in 200,000 births, also elevate the risk of CRC, and some genes responsible for these autosomal dominant disorders have been identified (
Table 19.1). Elucidation of the corresponding molecular defects serves not only in accurate molecular diagnosis, risk assessment, and disease prevention in affected families but also informs understanding of the considerably larger proportion of sporadic cases.
Familial Adenomatous Polyposis and the Central Importance of Wnt Signaling
In FAP, an autosomal dominant monogenic disorder that underlies about 0.5% of all CRCs, individuals develop hundreds to thousands of colonic polyps by their teens or early 20s, and the lifetime risk of progression to invasive cancer approaches 100%, with cancer diagnosed at a median age of 39. Extraintestinal manifestations include duodenal and gastric adenomas; congenital hypertrophy of the retinal pigmented epithelium; osteomas and mesenteric desmoid tumors in the Gardner syndrome variant
25; and less commonly, brain tumors in the Turcot syndrome variant,
26 cutaneous cysts, thyroid tumors, and adrenal adenomas. Although most features are benign, rare patients develop hepatoblastoma or thyroid cancer. Reflecting the similar regulation of small bowel and colonic epithelia, patients have a 5% to 10% risk of developing duodenal or ampullary adenocarcinoma, mandating close endoscopic monitoring of the upper intestine after prophylactic colectomy.
27The gene affected by mutations in this disorder, adenomatous polyposis coli (
APC) on chromosome 5q21, encodes a 2842-residue protein. Germline mutations occur throughout the locus but cluster in the 5′ half and exon 15,
28 mostly introducing stop codons or frame shifts that truncate the protein. Although a few mutations correlate with phenotypic severity or specific extraintestinal manifestations, identical mutations can produce different clinical features. In the attenuated APC (AAPC) variant, disease onset is delayed, individuals develop fewer colonic polyps or cancers, and mutations cluster in the extreme 5′ or 3′ ends of
APC exons.
29 The
I1307K allele, present in the Ashkenazi Jewish population, barely doubles the lifetime risk of CRC and does not affect APC protein function but replaces an (A)
3T(A)
4 coding sequence with an extended (A)
8 tract that is occasionally targeted for nearby truncating mutations.
30 Identification of an
APC mutation in a proband allows reliable testing of family members. Carriers should have screening colonoscopy annually after age 10, gastroduodenoscopy after age 25, and treatment with non-steroidal anti-inflammatory drugs to reduce the risk of progression to cancer.
31 Prophylactic colectomy is highly recommended, with subsequent monitoring of the rectal stump and other at-risk tissues.
The larger significance of the
APC gene derives from its somatic inactivation in about 80% of sporadic CRCs and early colorectal adenomas.
32 Indeed, somatic
APC mutations are found in tiny adenomas, containing few dysplastic glands. Attesting to the tumor suppressor function, tumors arising sporadically or in FAP patients show biallelic
APC gene inactivation and loss of heterozygosity, with one copy usually lost by deletion. Except for the small bowel,
APC mutations are rare in other cancers, including those in other digestive organs.
APC gene inactivation is a ratelimiting step for development of adenomas, and its designation as a gatekeeper gene in CRC is now well supported by knowledge of its cellular functions.
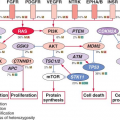
APC encodes several functional domains and proteins truncated by mutation that could in principle interfere with a wide range of cellular activities. Disruption of its known role in chromosome segregation might, for example, contribute to CIN.
33 However, attention on
APC centers rightfully on its control of the Wnt signaling pathway. About half the sporadic CRCs with intact
APC function carry activating point mutations in the
CTNNB1 gene,
34,
35 which encodes
β-catenin, a transcriptional effector of Wnt signaling. Moreover, acute loss of APC function in mice produces intestinal defects identical to those observed upon Wnt pathway activation.
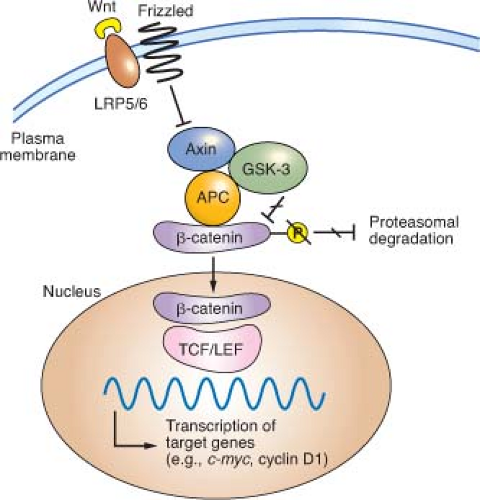
36The Wnt glycoproteins are secreted morphogens with diverse functions in development and homeostasis. In the absence of Wnt signaling, cells use a complex containing APC, Axin2, and other cytoplasmic proteins to promote phosphorylation, by casein kinase I and glycogen synthase kinase (GSK)-3
β, of several conserved serine and threonine residues in the
β-catenin N-terminus, thereby targeting
β-catenin for ubiquitin-mediated proteasomal degradation.
37 When Wnt ligands bind a surface protein complex that contains a member of the Frizzled protein family and the obligate coreceptor LRP5/6, they antagonize APC/Axin2 activity and thereby stabilize
β-catenin.
CTNNB1 mutations in CRC alter consensus residues for N-terminal phosphorylation and render the mutant protein resistant to
degradation. Thus, inactivating
APC or activating
CTNNB1 mutations, two alternative lesions in CRC, have the same effect: constitutive, Wnt-independent stabilization of
β-catenin (
Fig. 19.2). Accumulated
β-catenin translocates to the cell nucleus, where it acts as a transcriptional coactivator for the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors. Nuclear
β-catenin provides TCF/LEF proteins with an activating partner, resulting in transcription of target genes.
38 Of the four known TCF/LEF proteins, TCF4 is the most important in normal bowel epithelium and CRC.
34,
39 Among the many components of the Wnt signaling cascade, rare
AXIN2 mutations of uncertain significance are reported in MSI+ cases,
40 but mutations in CRC are otherwise found only in
APC and
CTNNB1.Although Wnt ligands signal in many tissues, intestinal homeostasis is particularly dependent on this pathway. Wnt signaling in the intestine is confined to proliferative crypt stem and progenitor cells. In mice, cycling crypt cells that express the surface marker LGR5 are far more susceptible to Wnt-induced transformation than their differentiated progeny, implying that CRC arises in a primitive stem or progenitor cell and not in mature descendants.
41 Moreover, the Wntdependent transcriptional program in CRC cell lines overlaps materially with that in intestinal crypts.
42 Wnt signaling is hence necessary for intestinal epithelial self-renewal, and constitutive, ligand-independent activation imposed by
APC or
CTNNB1 mutations induces and sustains adenomas. Among the diverse transcriptional targets of the TCF-
β-catenin complex identified to date,
CMYC seems especially important because its absence in mice abrogates the effects of acute APC loss in the intestine.
43,
44 Mice that lack CD44, another prominent Wnt-pathway target, develop fewer adenomas in an APC-deficient back-ground.
45 Gene expression profiling offers an expanded list of over 100 candidate transcriptional targets,
42,
46 but the individual significance of each will take many years to investigate.
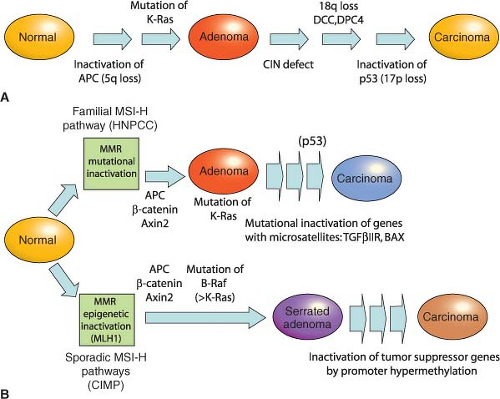
Hereditary Nonpolyposis Colorectal Cancer and the Role of DNA Mismatch Repair
HNPCC, an autosomal dominant disorder that confers a nearly 80% lifetime risk of developing CRC, usually before age 50, is estimated to account for 2% to 4% of all CRC cases in the United States.
47 Affected individuals do not lack intestinal polyps (nearly all CRCs, syndromic or sporadic, arise within adenomatous precursors) but develop many fewer colonic polyps than patients with FAP, a condition that must be excluded to satisfy criteria for diagnosis of HNPCC (
Table 19.2).
48 Cancers tend to develop in the ascending colon, and patients are further predisposed to develop tumors of the endometrium, small intestine, stomach, upper urothelium, ovary, biliary tract, and brain, a spectrum reflected in the revised Amsterdam II criteria (
Table 19.2). The lifetime risk of endometrial cancer, in particular, is 35% to 50% and that of urologic and ovarian cancers is 7% to 8%.
49 Cancers in HNPCC show pronounced variation in the lengths of microsatellite DNA sequences in tumors compared with unaffected tissues. Cancers showing such MSI at two or more among a panel of five mono- and dinucleotide tracts (BAT26, BAT25, D5S346, D2S123, and D17S250) carry the MSI-Hi designation. Most other CRCs harbor CIN and show microsatellite stability (MSS) or, in a small fraction, instability at only one of the five test tracts (MSI-Lo), a finding of uncertain significance.
HNPCC results from germline mutations in any of several genes that enable DNA mismatch repair (MMR), a proofreading process that corrects base-pair mismatches and short insertions and deletions in the normal course of DNA replication. MMR in mammalian cells is mediated by homologs of bacterial and yeast repair proteins: MutS homologs (MSH) 1-6, MutL homologs (MLH) 1-3, and PMS1 and PMS2. MLH1 and PMS2 are recruited to sites of DNA mismatch as a MutLα complex and in turn recruit MSH2-MSH6 (MutSα) or MSH2-MSH3 (MutS
β) heterodimers to sites of 1-bp or 2- to 4-bp errors, respectively. These proteins excise the strand that carries the mismatch, and they resynthesize and ligate the repaired DNA. Germline mutations in
MSH2, MLH1, and
MSH6 together explain more than 90% of kindreds
50,
51; the significance of mutations in other canonical MMR pathway genes,
PMS1 and
PMS2, is less certain.
52MSI-Hi colorectal cancers commonly show lymphocytic infiltrates, mucinous signet-ring differentiation, and a medullary growth pattern; Bethesda guidelines (
Table 19.2) combine clinical and phenotypic features to facilitate diagnosis of HNPCC.
53 When these criteria are met, tumor DNA should either be tested for MSI in a simple, PCR-based assay or by immunohistochemistry for absence of the commonly implicated MLH1, MSH2, and MSH6 proteins.
54 A positive result should prompt genetic testing for
MLH1, MSH2, or
MSH6 mutations; the personal and family history can predict the probability of identifying mutations.
55 Genetic testing identifies the mutant allele and carriers, allowing targeting of a recommendation for biannual colonoscopic screening between the ages of 20 and 40, with annual screening thereafter. Women should undergo annual endometrial evaluation soon after age 25, and carriers should consider prophylactic subtotal colectomy, hysterectomy, and oophorectomy.