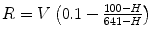Hematocrit, %
Blood volume (excluding citrate), mL
4.5
2.7
1.8
0.9
56
0.41
0.24
0.16
0.08
57
0.40
0.24
0.16
0.08
58
0.39
0.23
0.16
0.08
59
0.38
0.23
0.15
0.08
60
0.37
0.22
0.15
0.07
61
0.36
0.22
0.14
0.07
62
0.35
0.21
0.14
0.07
63
0.34
0.21
0.14
0.07
64
0.33
0.20
0.13
0.07
65
0.32
0.19
0.13
0.06
66
0.31
0.19
0.13
0.06
67
0.31
0.18
0.12
0.06
68
0.30
0.18
0.12
0.06
69
0.29
0.17
0.11
0.06
70
0.28
0.17
0.11
0.06
71
0.27
0.16
0.11
0.05
72
0.26
0.16
0.10
0.05
73
0.25
0.15
0.10
0.05
74
0.24
0.14
0.10
0.05
75
0.23
0.14
0.09
0.05
76
0.22
0.13
0.09
0.04
77
0.21
0.13
0.09
0.04
78
0.20
0.12
0.08
0.04
79
0.19
0.12
0.08
0.04
80
0.19
0.11
0.07
0.04
Another convenient approach is to remove a specified volume of citrate from a coagulation tube, then fill the tube with blood to the usual fill volume. Table 2.2 shows the volume of citrate to remove, as a function of hematocrit and tube size. For example, if a 3.0 mL tube (0.3 mL citrate and 2.7 mL blood) is to be used and the patient has a hematocrit of 60 %, first remove 0.09 mL citrate, leaving 0.21 mL citrate in the tube. Add enough blood to bring the total volume of blood and citrate to 3.0 mL (i.e., add 2.79 mL blood). The final citrate concentration in the specimen is 17 mmol/L of plasma plus citrate solution. Similarly, if the patient has a hematocrit of 70 %, first remove 0.14 mL citrate, leaving 0.16 mL citrate in the tube. Add enough blood to bring the total volume of blood and citrate to 3.0 mL (i.e., add 2.84 mL blood). Again, the final citrate concentration in the specimen is 17 mmol/L of plasma plus citrate solution. An advantage of this approach is that tubes are filled to their usual volume, circumventing the need to precisely measure the volume of blood to be added.
Table 2.2
Citrate reduction in specimens with high hematocrit, tubes filled to ideal volume
Hematocrit, % | Ideal fill volume of tube (blood + citrate), mL | |||
|---|---|---|---|---|
5.0 | 3.0 | 2.0 | 1.0 | |
56 | 0.12 | 0.07 | 0.05 | 0.02 |
57 | 0.13 | 0.08 | 0.05 | 0.03 |
58 | 0.14 | 0.08 | 0.06 | 0.03 |
59 | 0.15 | 0.09 | 0.06 | 0.03 |
60 | 0.16 | 0.09 | 0.06 | 0.03 |
61 | 0.16 | 0.10 | 0.07 | 0.03 |
62 | 0.17 | 0.10 | 0.07 | 0.03 |
63 | 0.18 | 0.11 | 0.07 | 0.04 |
64 | 0.19 | 0.11 | 0.08 | 0.04 |
65 | 0.20 | 0.12 | 0.08 | 0.04 |
66 | 0.20 | 0.12 | 0.08 | 0.04 |
67 | 0.21 | 0.13 | 0.09 | 0.04 |
68 | 0.22 | 0.13 | 0.09 | 0.04 |
69 | 0.23 | 0.14 | 0.09 | 0.05 |
70 | 0.24 | 0.14 | 0.09 | 0.05 |
71 | 0.25 | 0.15 | 0.10 | 0.05 |
72 | 0.25 | 0.15 | 0.10 | 0.05 |
73 | 0.26 | 0.16 | 0.10 | 0.05 |
74 | 0.27 | 0.16 | 0.11 | 0.05 |
75 | 0.28 | 0.17 | 0.11 | 0.06 |
76 | 0.29 | 0.17 | 0.12 | 0.06 |
77 | 0.30 | 0.18 | 0.12 | 0.06 |
78 | 0.30 | 0.18 | 0.12 | 0.06 |
79 | 0.31 | 0.19 | 0.13 | 0.06 |
80 | 0.32 | 0.19 | 0.13 | 0.06 |
It should be noted that the hematocrit threshold of 55 % appears conservative. Simple calculations show that if a tube containing 3.2 % sodium citrate is 90 % filled with blood with a hematocrit of 55 %, the final plasma specimen’s citrate concentration is 23.7 mmol/L. By inference, the maximum allowable citrate concentration in a plasma specimen under the CLSI guidelines is therefore 23.7 mmol/L. In a tube filled to its ideal volume (e.g., 3.0 mL blood plus citrate in a 3.0 mL tube), the citrate concentration is less than or equal to 23.7 mmol/L until the hematocrit is higher than 60 %, suggesting that a higher hematocrit threshold may be acceptable in completely filled tubes. Due to the difficulty, expense, and time delays involved in the preparation and use of citrate-reduced tubes, laboratories may wish to consider validating a hematocrit threshold higher than 55 % for citrate volume reduction.
2.3 Plastic vs. Glass Tubes
Over the past several years, the use of plastic specimen tubes throughout the laboratory has been on the rise for several reasons, including lower risk of breakage, which reduces biohazard exposure risk, and lighter weight, which reduces shipping and disposal expenses. Regulatory agencies have also strongly encouraged the use of plastic tubes and tube manufacturers have discontinued the production of many types of glass tubes.
Coagulation testing is known to be sensitive to the composition of specimen tubes [9], and historically, coagulation specimens have been collected in siliconized glass tubes to limit contact activation of clotting factors. Several studies comparing coagulation test results on plastic versus glass tubes found statistically significant differences in PT results between tube types, but in most cases the differences were deemed to be clinically insignificant [10, 11]; however, the differences were deemed potentially clinically significant in two studies [8, 12]. Rodgers and colleagues evaluated the effect of plastic tubes on esoteric coagulation tests, including factor assays, thrombin time, lupus anticoagulant, von Willebrand factor, protein C, protein S, APC resistance, and antithrombin, with normal donor plasma specimens. They found that the only test significantly affected was the thrombin time [13].
CLSI considers either glass or plastic tubes to be acceptable, provided that the inside surface is non-activating [1]. Nevertheless, before changing from one tube type to another, it is advisable for laboratories to evaluate the effects, if any, on their coagulation assays by conducting crossover studies using specimens with normal and abnormal results.
2.4 Collection Techniques
Several options are available for collecting blood specimens for coagulation testing, including venipuncture blood collection systems into evacuated tubes, winged needles and tubing, syringes, vascular access devices (VADs), and capillary specimens. Of these, the recommended method is venipuncture collection directly into tubes containing anticoagulant [1]. The other methods all have potential problems that may affect specimen quality. Winged collection systems have a length of tubing whose dead-space may result in under-filled tubes or activation of clotting factors or platelets if not properly managed. Syringe draws may result in activation of clotting factors when there is a delay in transferring blood from the syringe to the anticoagulated specimen tube. Syringe draws may also cause hemolysis if blood is drawn into or expelled from the syringe with too much force. VADs may lead to heparin contamination if the line is heparin-imbedded, has been flushed with heparin, or has had heparin administered through it. VADs may also lead to specimen dilution or partially clotted blood if the dead space is not managed appropriately. Capillary specimens are subject to activation of clotting factors if blood is not flowing freely, dilution with extracellular fluid if the skin puncture site is squeezed too vigorously, and hemolysis if the puncture site is overly “milked.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree