Fig. 5.1
Platelet satellitism and platelet clumping in a blood smear from a patient with EDTA-pseudothrombocytopenia (Courtesy Sherrie L. Perkins, MD, University of Utah Medical Center [Wright’s stain, oil immersion]) (From Rodgers [1], with permission)
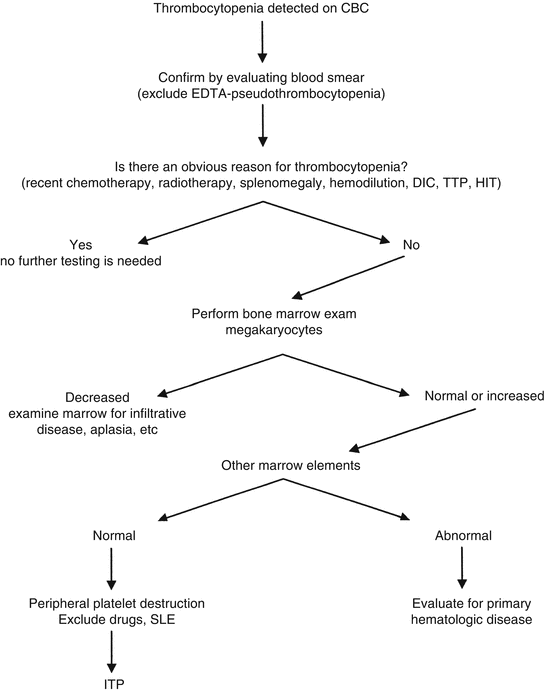
Fig. 5.2
A strategy to evaluate thrombocytopenia. Algorithm for evaluation of thrombocytopenia. Abbreviations: DIC disseminated intravascular coagulation, TTP thrombotic thrombocytopenic purpura, SLE systemic lupus erythematosus, HIT heparin-induced thrombocytopenia, ITP idiopathic thrombocytopenic purpura. The laboratory evaluation for DIC, TTP, etc. is discussed in Chaps. 8 and 9 (Modified from Rodgers [2], with permission)
In terms of hemostasis evaluation, one limitation of the CBC is that even though it is usually a reliable indicator of the platelet count, the CBC does not measure platelet function. The bleeding time test was originally thought to perform this function, but as discussed below, is not uniformly reliable in assessing platelet function.
5.3 The PT Assay
The PT assay has two purposes: to screen for inherited or acquired deficiencies in the extrinsic and common pathways of coagulation (Fig. 5.3) and to monitor oral anticoagulant therapy. The PT is affected by decreased levels of fibrinogen, prothrombin, factors V, VII, or X. Since 3 of the 5 coagulation factors measured by the PT are vitamin K-dependent proteins (prothrombin, factors VII and X), the PT assay is useful in detecting vitamin K deficiency from any cause including liver disease, malnutrition, or warfarin therapy. The PT does not measure factor XIII activity or components of the intrinsic pathway [3].
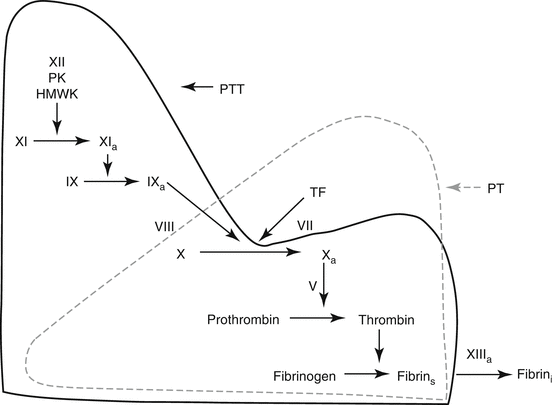

Fig. 5.3
The coagulation mechanism as measured by the PT and PTT assays. The PT assay measures the extrinsic (tissue factor) and common pathways, and is performed in the laboratory by mixing patient plasma with a commercial preparation of tissue factor and calcium (thromboplastin). This results in tissue factor-factor VII activation of factor X, then factor Xa – mediated conversion of prothrombin to thrombin. This reaction requires factor V as a cofactor. Thrombin converts fibrinogen to soluble fibrin, which polymerizes into fibrin strands, the endpoint detected by the coagulation instrument. Factor XIIIa cross-linking of fibrin is not measured by the PT assay. The PTT assay measures the intrinsic and common pathways, and is performed by adding patient plasma to the PTT reagent (contact activator). This preincubation step initiates contact activation of plasma in which factor XII and factor XI are activated in the presence of prekallikrein and high-molecular-weight kininogen. Factor XIa then activates factor IX to IXa. Calcium is then added to the sample. This results in factor IXa – mediated activation of factor X in the presence of the cofactor, factor VIII, with subsequent activation of prothrombin and fibrinogen are described above. As with the PT assay, the endpoint of the PTT assay is generation of polymerized fibrin strands, so that factor XIII activity is not measured by the PTT assay (Abbreviations: PK prekallikrein, HMWK high-molecular-weight kininogen, TF tissue factor, fibrin s soluble fibrin, fibrin i insoluble fibrin) (Modified from Rodgers [2], with permission)
The PT assay is performed by mixing patient plasma with thromboplastin. Thromboplastin is a commercial tissue factor/phospholipid/calcium preparation, which is derived either from animal tissue or from recombinant methods. Tissue factor in the thromboplastin preparation binds factor VII in patient plasma to initiate coagulation. The clotting time is measured in seconds using instruments with mechanical or photo-optical endpoints that detect fibrin formation [4]. Thromboplastin preparations can vary in their sensitivities, resulting in different clotting times. This is discussed further in Chap. 10. A typical PT reference range is 10–15 s. Most PT assays are automated, with the instrument adding reagents and patient plasma samples per protocol. Manual finger stick methods to measure PT/INR values are available and are discussed in Chap. 3.
In general, the PT assay is more sensitive in detecting low levels of factors VII and X than low levels of fibrinogen, prothrombin or factor V. In particular, different thromboplastin reagents may exhibit variable sensitivities to these factor deficiencies. Mild factor deficiency (i.e., 40–50 % of normal) may not be detected by many thromboplastin reagents. The PT assay is less affected by heparin than is the PTT assay, but therapeutic doses of unfractionated heparin will usually prolong the PT by a few seconds unless a heparin neutralizer is present in the PT reagent [3, 4].
Shortened PT values may result from either a poor quality venipuncture (activated sample), chronic disseminated intravascular coagulation (in vivo activation), or cold activation of the sample (in vitro activation from factor XII activation of factor VII) that occurs if the plasma sample is stored at cold temperatures (above freezing) for several hours. Administration of recombinant factor VIIa also will decrease the PT.
5.4 The PTT Assay
The PTT assay is useful for three reasons – as a screening test for inherited or acquired deficiencies of the intrinsic pathway (Fig. 5.3), to detect the lupus anticoagulant, and to monitor heparin therapy. The PTT is affected by decreased levels of intrinsic pathway components (factors VIII, IX, XI, XII, prekallikrein, and high-molecular-weight kininogen), as well as decreased levels of common pathway components (fibrinogen, prothrombin, factor V and X). Factors VII and XIII are not measured by the PTT assay [3].
To perform the PTT assay, patient plasma is preincubated with the PTT reagent (crude phospholipid and a surface-activating agent such as silica or kaolin). This preincubation initiates contact activation (intrinsic pathway activation) in which factors XII and XI are activated in the presence of cofactors, prekallikrein and high-molecular-weight kininogen. Factor XIa then converts factor IX to IXa. Calcium is then added to the preincubation mixture; this results in factors IXa/VIII activation of factor X, then factor Xa/V-mediated activation of prothrombin to thrombin followed by conversion of fibrinogen to soluble fibrin, the endpoint of the PTT assay [3, 4]. As with the PT assay, most PTT assays are automated.
The PTT is more sensitive to deficiencies of factors VIII and IX than to deficiencies of other intrinsic factors. Mild factor deficiency (i.e., 30–50 % of normal) may not be detected by most PTT reagents, but deficient levels of 10–20 % should be detected. The usual PTT reagent is less sensitive to factor IX than to factors VIII, XI, and XII. The ability to detect mild factor deficiency is reagent-dependent, and this is an important consideration when choosing the laboratory PTT reagent (see Chap. 4). For example, a hospital laboratory that evaluates a large population of bleeding disorder patients may prefer a PTT reagent that is more sensitive to detection of factor deficiency than to detection of lupus anticoagulants. In contrast, a hospital laboratory that evaluates large numbers of obstetrical patients may prefer a PTT reagent that has the opposite characteristics. The PTT may be affected by high levels of factor VIII, an acute-phase response protein; high factor VIII levels may mask co-existing mild intrinsic coagulation deficiencies. A typical PTT reference range is 25–36 s.
Shortened PTT values may result from a poor-quality venipuncture (activated sample), chronic disseminated intravascular coagulation (in vivo activation), or increased factor VIII levels.
It should be emphasized that the PT and PTT assays are screening tests, that normal PT/PTT results do not exclude a bleeding disorder, and that many patients with mild factor deficiency will have normal results for these assays [3].
5.5 The Mixing Test (Inhibitor Screen)
Abnormal PT or PTT results are due either to deficiency of a factor measured by the assay, or by an inhibitor such as an antibody or heparin. Uncommon inhibitors include fibrin degradation products and certain paraproteins. The mixing test is useful to distinguish between deficiency vs inhibitor, and mixing test results usually suggest subsequent test ordering. The most common mixing test protocol mixes patient plasma with normal plasma in a 1:1 ratio, followed by repeat assay of the PT or PTT immediately after mixing and repeated 1–2 hours later. Sample mixes that correct to normal at both intervals suggest that the original abnormal result was due to factor deficiency, while sample mixes that fail to correct to normal at one or both intervals suggest the presence of an inhibitory substance [3]. If heparin is suspected as the inhibitor, screening tests for the presence of heparin can be done with the thrombin time assay and reptilase assay (discussed below) or by direct assay of heparin or low-molecular-weight heparin using anti-factor Xa methods (see Chap. 10).
The mixing test is most useful for evaluating prolonged PTT results. Almost all prolonged PT samples result from factor deficiency, and the mixing test is less useful in this circumstance.
5.6 The Thrombin Time Assay
The thrombin time (TT) measures the conversion of fibrinogen to fibrin. It is performed by the addition of purified thrombin to patient plasma; the resulting clotting time is a function of fibrinogen concentration and activity. The TT is a screening test for the presence of heparin in a plasma sample. Other causes for a prolonged thrombin time include quantitative deficiency of fibrinogen, qualitative abnormality of fibrinogen (dysfibrinogen), elevated levels of fibrin degradation products (FDP), the presence of certain paraproteins, and markedly increased levels of fibrinogen (>5 g/L) [3].
If heparin is suspected as the cause of a prolonged TT, confirmation of heparin presence can be made using heparin assays (see anti-Xa assays in Chap. 10) or by using the reptilase clotting time that also measures the conversion of fibrinogen to fibrin. The reptilase clotting time is not affected by heparin. Therefore, a plasma sample with a prolonged TT, but a normal reptilase clotting time indicates the presence of heparin.
For hypofibrinogenemia to prolong a TT, the fibrinogen value will usually be <0.7–1 g/L. If the thrombin concentration in the TT assay is more than 3 U/mL, the assay will be less sensitive in detecting abnormalities.
5.7 Fibrinogen Assays
Fibrinogen is a heterodimeric molecule, with each half of the molecule composed of three polypeptide chains – Aα, Bβ and γ. It is acted upon by thrombin to produce fibrin monomers that polymerize to form fibrin strands, and ultimately a fibrin clot. Circulating fibrinogen molecules are structurally heterogeneous, and not all fibrinogen molecules are capable of participating in clot formation. Therefore antigenic assays and clot-based assays may return different results depending upon the composition of fibrinogen molecules in a specific patient sample [5]. Only clottable fibrinogen is of interest for the purpose of hemostasis screening.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree