Fig. 6.1
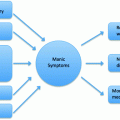
Potential interactive and non-interactive effects of age and bipolar disease (used with permission from Weisenbach et al. [4])
In their cross-sectional study of young and late middle-aged patients with BPD (in the euthymic state) and healthy controls, Weisenbach et al. [4] demonstrated that late middle-aged adults with BPD have especially poor performance (relative to young adults and same-age healthy peers) in the domains of emotion processing, processing speed, and aspects of executive functioning (i.e., verbal fluency, attentional shifting, and interference resolution), with some effects demonstrating an acceleration of the aging process in BPD (similar performance between the two young groups, but poorer performance in the older BPD group relative to same-age controls), and others suggesting a compounded effect of BPD during aging (poorer performance in the young BPD group relative to same-age controls, but incrementally poorer performance in the older BPD group relative to same-age controls). At the same time, verbal memory and other aspects of executive functioning, such as inhibitory control, conceptual reasoning, and set shifting were impacted only by age, regardless of disease status, while fine motor skills and visual memory skills were affected by aging and disease independently. It is important to consider that the older groups in this study were in their mid-50s. Given that more severe cognitive changes are seen in the oldest old during the normal aging process (i.e., a nonlinear trajectory of age-related cognitive changes, [3]) and cognitive changes in BPD have been shown to accelerate after age 65 [6], it is not clear whether these findings would extend to the oldest old with BPD. Other case–control studies of older euthymic BPD patients also demonstrate poorer performance relative to healthy, similarly aged peers across multiple cognitive domains, including processing speed, language, visuomotor skills, episodic memory, working memory, and processing speed [7–10]. At the same time, a study by Sajatovic et al. [11] found no differences in performance among young (40 years and younger) versus older (60 years and older) patients with BPD, when using age-corrected normative scores.
Longitudinal studies of BPD in middle and late life have demonstrated somewhat variable findings. Delaloye et al. [12] found no differences in the trajectory of cognitive changes over two years between BPD and controls. In a different sample, Depp et al. [13] followed patients and controls for up to 3 years and demonstrated greater variability on a measure of global cognition in the BPD patients. Gildengers et al. [14] found that global cognition was poorer at baseline in BPD, relative to controls, and declined more rapidly over 3 years. In a separate study, with a different sample and a more comprehensive neuropsychological battery, Gildengers et al. [15] found poorer cognitive function across all domains, as well as worse performance on instrumental activities of daily living among BPD patients relative to controls at baseline and at 2-year follow-up. However, there was no evidence for accelerated decline in the BPD group across the 2-year period. In summary, studies demonstrate cognitive problems across a multitude of domains in BPD that can occur early during the disease course and may be compounded by and/or accelerated with the aging process, although findings are mixed in this regard.
Clinical characteristics of OABD may also moderate the extent of cognitive impairment observed. First, disease state and greater symptom severity have been shown to impact the severity of cognitive problems, with poorer cognition occurring during manic and depressive, relative to euthymic phases. Having a later onset of BPD may also confer greater risk of cognitive difficulties [16]. After controlling for age, years of illness was not found to be associated with cognition in the study by Weisenbach et al. [4], though there have been studies demonstrating a significant relationship between cognition and number of episodes in patient groups of a wide age range [17, 18] particularly when comparing those with one or two episodes to those with three or more episodes [19].
6.3 Potential Mediators of Cognitive Impairment in OABD
There are likely a number of factors that contribute to cognitive problems in BPD and OABD. While a comprehensive discussion of these variables as they relate to BPD, in general, is covered elsewhere in this volume, we will briefly discuss some of the primary areas currently under investigation in the field. We also refer the reader to a recent manuscript by Sajatovic et al. [11] that provides an excellent overview of possible mediators of cognitive impairment in OABD.
First, medical comorbidities are more common among individuals with BPD than in the general population, including cardiovascular disease, respiratory disorders, type II diabetes mellitus, hypertension, and coronary artery disease, among others, with the majority of patients having three to four comorbid medical conditions [20]. Lifestyle factors, including substance abuse, smoking, poor diet, and metabolic dysregulation as a consequence of psychotropic medication use, contribute to the high prevalence of medical comorbidities in this population [21]. Cognitive impairment has been associated with the presence of medical comorbidities in OABD. A recent study found that hypertension, metabolic syndrome, abdominal obesity, and hyperglycemia were nominally associated with poorer performance in a multitude of cognitive domains, though only hypertension was statistically significant. In at least two studies, greater medical comorbidities have been associated with poorer daily functioning [15, 22], though this is partially accounted for by poorer cognitive functioning [15].
Second, acute side effects of psychotropic medications, in addition to effects of long-term use of psychotropic medications, are thought to contribute to cognitive dysfunction in OABD. With regard to more acute effects, sedating properties of GABA-ergic medications, such as benzodiazepines, are associated with cognitive blunting [23]. Anticholinergic medications also impact cognition to a greater extent than medications without anticholinergic effects [24, 25]. Lithium is known to have anticholinergic properties, for instance, which may explain initial adverse impact on cognition [26]. The longer-term impact of lithium on cognitive functioning is less clear. There have been a handful of longitudinal studies (all limited to 10 years or less) on the effects of long-term lithium use on cognition, though none have revealed findings suggesting that lithium accelerates cognitive decline in BPD [27–30]. In fact, lithium may have neurotrophic/neuroprotective effects [31–33] that can preserve cognition [34, 35] and even prevent or delay the onset of dementia [36].
Third, there have been a number of neuroimaging studies investigating the role of white matter degeneration and gray matter atrophy in BPD, which could underlie cognitive deficits in OABD. Haller et al. [37] found decreased white matter integrity in the ventral portion of the corpus callosum, as well as reduced gray matter density in limbic, subcortical, and prefrontal regions among 19 older BPD patients relative to 47 controls. Findings have been mixed, however. For example, Rej et al. [9] reported increased white matter hyperintensities (WMHs) among controls, relative to OABD, and no differences in total gray matter or hippocampal volume. WMHs were not correlated with cognitive performance in the BPD group. Delaloye et al. [12] found no differences between OABD and controls in the trajectory of changes of gray and white matters over two years. Neuroimaging studies of OABD are limited, and sample sizes are small; thus, more research in this area is needed before making any firm conclusions about the relationship between neurodegeneration and cognitive difficulties in OABD.
Other mechanisms underlying neurocognitive decline in OABD include neurochemical dysregulation, oxidative stress, neuroinflammatory processes, mitochondrial dysfunction, and neurotrophic factors. Dysregulation of the dopaminergic and glutamatergic systems, such as occurring in BPD, can lead to oxidative stress [38, 39], which has been associated with cognitive decline during normal aging [40]. Neuroinflammatory processes are also likely implicated in cognitive decline in the context of BPD. Increased levels of pro-inflammatory cytokines have been demonstrated in BPD [41] and have been associated with cognitive decline both in animal and in human models [42, 43]. Mitochondrial dysfunction, which impairs oxidative energy generation, is increasingly being recognized as relevant to BPD [11] and may also be responsible, at least in part, for the cognitive decline observed in BPD. Finally, neurotrophic factors (i.e., BDNF) have been shown to be decreased, particularly during active episodes [44, 45], and may be reduced more chronically in the later stages of BPD [46]. A recent meta-analysis of ten studies found that low levels of BDNF in BPD were negatively associated with cognitive performance [42]. Figure 6.2 illustrates possible sources of cognitive impairment in OABD.
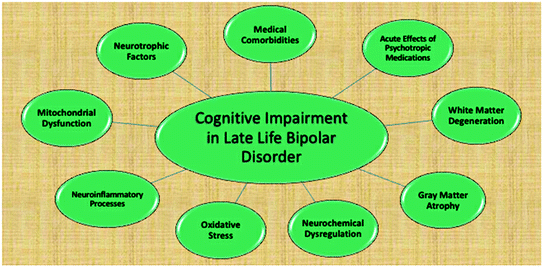

Fig. 6.2
Cognitive impairment in late-life bipolar disorder. Displays possible sources of cognitive impairment in OABD
6.4 Management of Cognitive Impairment in OABD
6.4.1 Screening
Because neuropsychological evaluations are time-consuming and costly and require the expertise of a clinical neuropsychologist, the use of screening methods for populations at high risk of cognitive impairment/decline is imperative. In fact, a combination of poor sensitivity and specificity of screening tests, along with the difficulty acquiring a test that comprehensively assesses cognitive domains, impedes identification of cognitive decline in the early stages [47]. In an effort to prevent delays in detecting cognitive decline, many researchers have conducted investigations as to the sensitivity and specificity of neuropsychological measures, both direct and indirect, for the detection of cognitive impairment (Tables 6.1 and 6.2). Thus, cognitive screening measures need to be easily administered independent of language, culture, and education and must be well tolerated by patients. Cognitive screening instruments can detect the presence of cognitive decline that should be further evaluated through comprehensive neuropsychological evaluation. Ideal cognitive screeners have high sensitivity and specificity, high concurrent and predictive validity, as well as ease of scoring and good inter-rater and test–retest reliability [47]. It is important to keep in mind that cognitive screening should not be used to diagnose mild or major neurocognitive disorders, as they are designed to indicate the presence/absence of cognitive impairment, as opposed to provide the kind of comprehensive evaluation that is necessary for arriving at specific diagnoses.
Table 6.1
Direct screening measures
Test | Sensitivity | Specificity | Cost | Location |
|---|---|---|---|---|
MMSE | 63–69 %a (dementia) | 90–96 %a (dementia) | $68.00/pkg 50 | |
Six-Item Screener (≥1 error) | 100 %b (dementia) | 38.4 %b (dementia) | FREE | |
Mini-Cog | 76–99 %c (dementia) | 83–93 %c (dementia) | FREE | |
Clock drawing task (CLOX-I) | 90 % (AD)d 75 % (MCI)d | 75 %(AD)d 50 %(MCI)d | FREE | |
Category fluency | 88 %e (AD) | 96 %e(AD) | FREE | N/A |
MoCA | 90 % (MCI)f 100 % (AD)f | 87 %f | FREE |
Table 6.2
Indirect screening measures
Test | Sensitivity | Specificity | Cost | Location |
|---|---|---|---|---|
Informant Questionnaire | 80–90 %a (dementia) | 80–90 %a (dementia) | FREE | |
AD8 | 84 %b (dementia) | 80 %b (dementia) | FREE |
Direct screening measures are those administered by the clinician. These measures need to be able to quickly assess patients in a clinic and at bedside, and must be sensitive, specific, and efficient [47]. The measures included below are some of the more widely used and popular screening measures, but by no means represent an exhaustive list.
6.4.1.1 Mini-Mental State Examination
The Mini-Mental State Examination [48] (MMSE) is a 30-point measure of global cognitive functioning that takes roughly 5–10 min to administer and is commonly used to track the cognitive changes. The MMSE has been shown to be sensitive for screening of dementia, but is less sensitive for detection of mild neurocognitive disorder (MND), formerly known as mild cognitive impairment. Approximately 80 % of individuals with MCI score above 26 points, which is higher than the most typically used cutoff of 24 points [49]. Relatedly, Spering et al. [50] found that the MMSE does not adequately discriminate between patients with normal versus impaired memory, or visuospatial performance. In addition, while the MMSE includes items assessing orientation, attention, memory, language, and visuospatial skills, it does not formally evaluate executive functions, which are commonly affected in late-life mood disorder [49]. It is also important to consider that MMSE scores are positively associated with education level and negatively correlated with age. Low education and ethnicity, which often co-occur, lead to a trend of scores among Caucasians to be typically higher than scores of other ethnic groups [50]. Normative data for age and educational level are available [51], and while the MMSE is limited in its ability to detect MCI, when used in conjunction with tests that measure executive functioning, such as the clock drawing test (CDT), it yields a better representation of the individual’s cognitive status [52].
6.4.1.2 Montreal Cognitive Assessment
The Montreal Cognitive Assessment [49] (MoCA) is a 30-point screening measure assessing visuospatial skills, executive functioning, memory, naming, attention, language, delayed recall, abstraction, and orientation and was developed to address the limitations of the MMSE for accurate detection of MCI and dementia [53]. To account for the impact of education on performance, the MoCA adds one point if education is 12 years or less. Additionally, the MoCA, when compared to the MMSE, places more emphasis on tasks of frontal executive functioning and attention deficits found in the beginning stages of many non-AD dementias and in non-amnestic MCI, in addition to late-life mood disorders. This may account for its higher sensitivity for detecting non-AD dementias and non-amnesic MCI [54]. The suggested cutoff score for cognitive impairment on the MoCA is <26. In cases where the MMSE has been given as an initial screen and yielded normal results when functional impairments are evident, the MoCA should be administered because 100 % of individuals with mild AD had an abnormal MoCA score. When patients present with no functional impairments but complain of cognitive impairment, one should begin the screening with a MoCA because in this case the MMSE would likely yield a normal result [49].
6.4.1.3 Mini-Cog
The Mini-Cog [55] is a 3–5 min cognitive screener comprised of two parts, a delayed three-word recall task and the CDT. This screener was designed to eliminate the effects of educational, linguistic, or cultural biases due to the brief memory task and clock drawing task that are not highly affected by the same biases that are commonly observed with use of screeners such as the MMSE, where common language, culture, and education impact ability [56]. Patients with cognitive impairment will draw an abnormal clock and recall only 1 or 2 of the 3 words after a delay, or will recall no words and have a normal clock (i.e., a positive result [55]). Individuals with a positive result should be evaluated further.
6.4.1.4 Six-Item Screener
The Six-Item Screener [57] (SIS) was developed for use in research to examine large numbers of individuals in a short amount of time and assesses orientation to date and 3-item short-delayed recall. The measure takes 1–2 min to administer in comparison with others, whose administration time is usually 7 min or longer, and can be administered over the phone. The test is scored based on the number of errors made with the sensitivity for dementia being 96.8 % and specificity being 53.3 % for one or more errors, with lower sensitivity (89.6 %) but higher specificity (79.4) for two or more errors [57]. The SIS sensitivity is comparable to the MMSE for the detection of possible dementia and could be used as a first-stage screen for cognitive impairment.
6.4.1.5 Clock Drawing Test
The clock drawing test (CDT) provides a simple and reliable screening measure for cognitive impairment when patients are asked to draw a clock with the hands at 10 past 11. Correctly placing the perceptual features of the clock requires intact frontal executive activity in order to inhibit the tendency to be swayed by placing the hands at the “10” as opposed to 10 min after. In addition to inhibition, it requires (a) auditory comprehension, (b) planning, (c) visual memory and reconstruction, (d) motor programming, (e) numerical knowledge, (f) abstract thinking, and (g) concentration [47]. Practical limitations of CDTs are that there is not one way to administer and score this task. Regarding psychometric properties, the CDT has been shown to have a mean sensitivity of 85 % as well as good inter-rater reliability and good predictive and concurrent validity [58]. Different versions of administration and scoring of CDTs are available. Table 6.1 lists information for CLOX-I [52]. Unfortunately, while CDTs are widely accepted and used, they are traditionally seen as visuospatial and executive functioning tasks, which would not be as useful for detecting amnestic cognitive impairment, for example [52]. While the test is sensitive to detecting cognitive change, it is best used in conjunction with other screening measures, such as the MoCA, and should not be used alone for diagnostic purposes.
6.4.1.6 Category Fluency
Category fluency tasks are comprised of both semantic and phonemic tests of verbal fluency. These measures assess both language and executive functioning and are ideal for bedside administration, as they take roughly 3 min to administer (with some variation depending upon versions used). With regard to screening for cognitive impairment, research has shown that patients’ abilities on semantic fluency tasks decline as cognitive impairments worsen [59]. An individual’s semantic fluency score is typically lower than the phonemic fluency in the case of amnestic MCI compared to controls and is positively correlated with memory performance [60]. This research suggests that clinicians should be cognizant of the differences in performance between fluency tasks to help determine whether further neuropsychological evaluation for cognitive impairment is warranted. Because fluency measures are limited in the cognitive abilities upon which they draw, they should be used in conjunction with other more comprehensive screening measures.
When assessing individuals with cognitive impairments, it can be helpful to gather collateral information, which can be done quickly using informant questionnaires. Such information can indicate whether the cognitive problems observed currently are long-standing in nature, or represent a significant change for the individual, possibly reflecting a neurodegenerative process. Individuals who have frequent exposure to the patient, including the patient’s spouse and child, etc., have been shown to consistently and accurately report symptoms regarding orientation, memory, problem solving, and judgment when correlated to the performance on semi-structured interviews. Collateral information has shown to assist in delineating normal aging from dementia, since it can provide an indication of the patient’s baseline level of functioning [61]. Furthermore, subjective reports of mild cognitive impairments often lead to an underreporting of deficits [62]. With this in mind, informant measures such as the IQ-CODE [62] and the AD8 [63] can promote accurate screening for referrals and ultimately diagnosis. Unfortunately, many caregivers of individuals with cognitive impairments have difficulty coping with lifestyle changes and can be at risk of developing depression due to feeling overburdened [64]. Pressure to keep patients safe, healthy, and well places many under immense amounts of stress due to caring for an individual with cognitive impairment, causing subjective feelings and mood to negatively impact the reporting of deficits the patient has incurred, contributing to possible over-reporting of symptoms [65]. Informant questionnaires should be used in conjunction with performance-based screening measures, such as those described above. While we discuss informant measures below in further detail, there are many other measures that can be utilized to collect data, such as the Measurement of Everyday Cognition Scale (E-Cog); [66] that are not discussed here.
6.4.1.7 Informant Questionnaire on Cognitive Decline
The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) is a 26-item informant interview used as a measure of functional change [62]. Total scores range from 26 to 130 and are achieved from summing scores ranging from 1 (has become much better) to 5 (has become much worse), with higher scores representing greater decline in cognitive functioning [67]. This measure has high internal consistency and high test–retest reliability and has been shown to be sensitive in the detection of dementia [62].
6.4.1.8 AD8
The AD8 is a 3-min assessment used to determine intra-individual cognitive changes. It consists of eight questions that are summed (i.e., plus one) if the informant endorses “yes, there has been a change in cognitive functioning,” and yields a maximum score of 8 that indicates over the past several years there has been a noticeable decline in thinking and memory problems [68]. This screening measure is sensitive for detecting early cognitive decline, as well as differentiating between amnesic and non-amnesic forms of dementia, and performs well independently of age, race, education level, sex, and MMSE scores. The AD8 functions as an accurate brief screening measure that has strong internal consistency, concurrent validity, construct validity, and inter-rater and intermodal reliability [68] and can be used to assist in detecting changes in individuals’ cognitive impairment.
6.4.2 Referring for Neuropsychological Assessment
Referral for comprehensive neuropsychological evaluation should be made when reversible causes of cognitive impairment, such as metabolic changes causing delirium, have been ruled out, and a positive result is indicated on cognitive screen. The screening measure(s) that the clinician chooses should be first based upon its sensitivity and specificity in detecting cognitive impairment, as described in the sections above, but is also realistically based upon how much time is available to administer the measure. While the MoCA, for example, is a comprehensive screening measure and can detect milder forms of cognitive impairment than the MMSE or Mini-Cog, it can take up to 20 min to administer. The Mini-Cog was developed to be a much briefer screen that is often feasible to administer in a busy office environment. One strategy would be to administer lengthier screens, such as the MoCA, when families (via informant screens completed in the waiting room) and/or patients are complaining of cognitive changes but pass a briefer screen, such as the Mini-Cog.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree