Fig. 3.1
Imaging from research has shown decrease in the volume of gray matter in patients with bipolar disorder in the right cudate, globus palidus, lateral ventricles, insula, and amygdale. White matter hyperintensities have been observed in the corpus callosum, parietal, frontal, and subcortical basal ganglia
The temporal lobe is another brain region of interest for researchers because of its cortical and subcortical connections to areas of the brain that regulate emotion, mood, and memory [21]. Jones et al. [22] reported increased temporal lobe volumes in adult patients with BD. In another study, amygdala volumes were found to be significantly larger in the bipolar group (average age 50.2 ± 12.7) compared to normal and schizophrenic groups [23].
A relatively recent review of MRI imaging studies from 2009 also showed whole brain and prefrontal lobe volume reductions and increased volume of the globus pallidus and lateral ventricles [24]. Wijeratne et al. [25] recently demonstrated that older patients with BD had smaller amygdala and hippocampal volumes, and that the latter was negatively associated with the duration of manic and depressive episodes in these individuals. Other studies have discovered decreased gray matter concentration in the right anterior insula, nucleus accumbens, ventral putamen, and frontal orbital cortex. In contrast, in a study comparing 71 older BD individuals to 82 controls, the OABD group showed no significant age-associated volumetric changes in gray matter [26]. Nevertheless, despite some conflicting studies, the current evidence suggests that bipolar disorder does involve a reduction in brain volume, though the pathophysiology of this change requires further elucidation.
3.4 Diffusion Tensor Imaging
Diffusion tensor imaging (DTI)/diffusion MRI, and the subsequent calculation of fractional anisotropy (FA), demonstrates microstructural alterations of white matter in Older Age Bipolar Disorder. Diffusion MRI permits the in vivo mapping of the diffusion process of molecules (mainly water) in biological tissues. Its main application is in the study of neurological disorders such as stroke, as it can reveal aberrations in the structure of white matter. The primary outcome measure of DTI is fractional anisotropy (FA). FA describes the degree of directionality in the diffusion process and can be calculated to analyze the integrity of these white matter tracts. A greater value of FA correlates with greater integrity of these tracts. Other parameters that measure regional white matter microstructural integrity include axial diffusivity and radial diffusivity, though FA seemed to be the most utilized among studies.
One study utilized FA to determine whether there were different tracts involved in BDI and BDII. The results suggested a difference in FA in BDII subjects when compared to BDI and healthy controls within the inferior longitudinal fasciculus, and common to both BDI and BDII there were changes observed in the internal capsule, cortico-spinal tract and cerebellum (from demyelination or axonal damage) [27].
Interestingly, Haller et al. [28] studied a geriatric population of 19 individuals with BD [mean age = 68.5] and 47 controls [mean age = 69.7]. In subjects with BD, decreases in gray matter concentration in limbic areas, reductions in fiber tract coherence in the corpus callosum region, and a trend relating decreased FA values to illness duration were some of the important findings of the study. A more recent study by Toteja et al. also reported similar results. In this study, DTI was performed on 57 patients with BD and 57 sex- and age-matched controls. The study observed that in the BD group, there were age-associated increases in mean diffusivity in the corpus callosum [29]. Impairment in the integrity of the corpus callosum has been specifically implicated in late-life bipolar disorder [30].
3.5 Magnetic Resonance Spectroscopy
Of note, in a lithium-7 magnetic resonance spectroscopy (MRS) study focused on the corpus callosum, Forester et al. [31] found that the relationship between brain and serum lithium levels was moderated by age such that serum lithium levels were not associated with brain levels in patients 50 years of age or older. Furthermore, higher brain lithium levels were associated with frontal lobe dysfunction. Thus, neurochemical abnormalities and the disruption in these white matter tracts and the regions of the brain they connect are thought to be a contributor to the mood dysregulation that occurs in bipolar disorder [27].
3.6 Functional Neuroimaging: PET, SPECT, and fMRI
In addition to structural neuroimaging analyses, some studies in BD research have utilized functional neuroimaging techniques such as positron emission topography (PET), single photon emission topography (SPECT), and functional MRI (fMRI). However, compared to the literature examining gray and white matter changes, functional studies in BD, especially in OABD, are quite sparse. In a comprehensive review of neuroimaging findings in bipolar disorder, Phillips and Swartz [32] suggest that BD is characterized by state-specific alterations in circuits underlying emotion processing and emotion regulation (amygdala, insula, orbitofrontal cortex, dorsolateral prefrontal cortex). Additionally, they report that in resting-state fMRI studies, there is a pattern of altered intrinsic connectivity in fronto-temporal-striatal circuits. Their review also highlights a critical gap in the literature by the fact that to date, there are no published functional imaging studies in OABD.
3.7 Bipolar Disorder as a Neuroprogressive Disorder
Stemming from these abnormal imaging findings is the concept of neuroprogression that has been proposed for bipolar disorder and OABD. Neuroprogression is a relatively new term that describes the detrimental changes (defined as increase in proinflammatory cytokines, oxidative stress products, decrease in anti-inflammatory cytokines, decrease in treatment response, and decrease in cognitive and functioning performance) that occur along the natural history of bipolar disorder as it progresses [33]. Though more studies are needed, imaging has increased our understanding of the structural and connective changes in the brain that have been observed in OABD [34]. One study by Gildengers and coworkers, for example, studied fifty-four adults with BD [mean age = 64.4 years] for the purpose of investigating whether BD is a neuroprogressive disorder. Using MR imaging, the study found that lower total gray matter volume was related to longer duration of bipolar illness, even when controlling for age [35]. The findings of this study provide evidence for the theory of neuroprogression in older adults with BD.
The neuroprogression hypothesis postulates that neurochemical dysregulation, neuroinflammation, oxidative stress, and mitochondrial dysfunction contribute to changes in brain structure and function (Fig. 3.2). For example, excessive dopamine and glutamate neurotransmission may lead to a decrease in brain derived neurotrophic factor (BDNF) and secondary structural changes in gray and white matter [13]. Neuroprogression may also explain the clinical observation of decreased treatment response and increased risk of future recurring episodes for individuals with severe bipolar disorder. Researchers also posit that with neuroprogression, the different processes that cause these structural and functional changes may represent stages with the progression of BD.
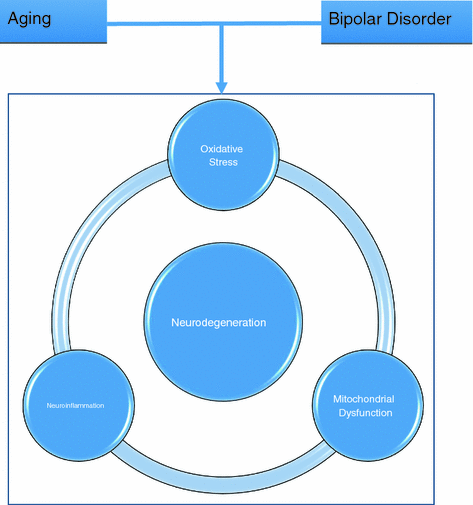

Fig. 3.2
Schematic demonstrating how aging and bipolar disorder contribute to linked processes underlying neurodegeneration: oxidative stress, mitochondrial dysfunction, and neuroinflammation
Some neuroimaging studies have demonstrated neuroprotective effects of various treatments for bipolar disorder including lithium, omega 3 fatty acids, statins, and anti-inflammatory medications. Giakoumatos et al. studied patient populations with BD that were lithium-free versus lithium-treated. Findings suggest that patients without lithium treatment had substantially smaller hippocampal subfield volumes than patients treated with Lithium. Based on this evidence, the authors postulated that lithium may counteract the gray matter changes and cortical thickness changes found in bipolar disorder [36, 37].
3.7.1 Biomarkers of Oxidative Stress and Mitochondrial Dysfunction
Oxidative stress and mitochondrial dysfunction have been identified as key components in the mechanisms underlying neuroprogression in BD [38]. Oxidative stress arises from the imbalance in generating reactive oxygen species (ROS) and the body’s ability to remove or repair ROS. It is believed that oxidative stress is associated with systemic changes involved in the aging process [39]. Oxidative stress has been implicated in the pathophysiology of BD and has been suggested as a possible biomarker involved in different stages of the illness. In one of the first meta-analyses of oxidative stress and bipolar disorder, Andreazza et al. [40] found that specific biomarkers of oxidative stress, thiobarbituric acid reactive substance (TBARS, a marker of lipid peroxidation), and nitric oxide (NO) were significantly elevated in patients with bipolar disorder. More specifically, elevations in TBARS appear to be related to the manic state. Tsai and Huang [41] showed that elevated TBARS levels in manic patients were reduced after treatment. Additionally, NO has been shown to correlate with the number of manic episodes in euthymic BD patients [42]. Furthermore, late-stage bipolar disorder (at least 10 years of illness) has been associated with an increased activity of the antioxidant enzymes glutathione reductase and glutathione S-transferase [43]. The evidence of oxidative stress in BD appears to persist into later life. These findings have been replicated in a number of studies, and recent meta-analysis identified lipid peroxidation, DNA/RNA damage, and NO as significantly increased in BD patients compared to controls [44].
Another pathophysiological process that could contribute to neuroprogression in bipolar disorder and the neuroimaging findings previously described is mitochondrial dysfunction. Mitochondrial dysfunction is a major source of oxidative stress and has been implicated in the pathophysiology of bipolar disorder. Specifically, higher levels of mitochondrial DNA oxidative damage have been demonstrated in BD [45]. Furthermore, BD patients exhibit abnormalities in peripheral mitochondrial morphology and distribution. Specifically, BD patients were found to have smaller mitochondria that were more likely located in the perinuclear region as opposed to distal processes [46]. Researchers have used innovative neuroimaging techniques to probe in vivo neuronal mitochondrial function in BD. Using magnetic resonance spectroscopy (MRS), studies have shown alterations in metabolites associated with neuronal energetics such as pH changes, reductions in high-energy phosphates, and n-acetyl aspartate (a marker of mitochondrial function) [47–50].
The notion that oxidative damage is a key component in the pathophysiology of BD has led to a number of interesting treatment trials targeting oxidative damage and mitochondrial dysfunction. Since cysteine is an important precursor to glutathione, an antioxidant, it was thought that n-acetylcysteine (NAC) could be beneficial in the treatment of bipolar depression. In a series of studies, Berk et al. [51, 52] demonstrated that NAC was associated with a decrease in depressive symptoms in bipolar patients. Of relevance to older patients with BD, it was shown that medical comorbidity moderates the benefits of NAC in bipolar depression [53]. In OABD, 4 weeks of treatment with CoEnzyme Q10, an effective antioxidant known to enhance mitochondrial function, improved depression severity scores [48, 54].
In addition to novel treatments for BD, the notion of oxidative stress has implications for the effects of existing treatments for BD. For example, in tissue culture, lithium has been shown to increase mitochondrial oxidative phosphorylation activity, suggesting a neuroprotective effect [55]. Lithium also increases mitochondrial complex I activity in the context of bipolar depression [56]. The role of oxidative stress has not been limited to lithium. In a study of the treatment response in OABD, Gildengers et al. [57] found that lamotrigine was most effective in patients with high cardiometabolic risk, a risk factor associated with damage from oxidative stress.
3.8 Clinical Implications Present and Future
The clinical implications from recent studies of the neurobiology of bipolar disorder in late-life suggest novel targets for intervention, as well as alternative mechanisms of actions for existing treatments as described above. Future implications of these studies indicate an opportunity to personalize BD treatments according to specific clinical profiles. For example, BD patients with significant medical comorbidities may benefit from adjunctive treatments that target oxidative stress, such as NAC. Additionally, neuroimaging techniques like MRS could be used to track treatment response to antioxidants measuring metabolites that reflect mitochondrial function. As highlighted by the work of Forester et al. [31], MRS could also be used to determine brain levels of lithium, which may be more sensitive in detecting lithium toxicity than current practice focusing on serum lithium levels. This notion is of particular relevance for the clinical vignette at the beginning of the chapter since serum lithium levels may not reliably reflect brain levels in older patients.
Furthermore, an expanded knowledge base of neural networks that are characterized by neuroimaging methods and involved in BD could guide non-invasive neuromodulatory treatments, such as transcranial magnetic stimulation or transcranial current stimulation, that are designed to engage or enhance specific brain circuits.
3.9 Summary and Conclusion
The advent of neuroimaging and its increasing sophistication has helped to bring about a better understanding of the structural and functional changes that occur in bipolar disorder. Many of the studies reviewed highlighted the potential importance of neuroimaging for bipolar disorder particularly as a conduit for discovering clinically relevant biomarkers of disease state and predictors of treatment response. However, the current clinical role for neuroimaging remains yet to be clearly defined. Neuroimaging research to date in OABD is limited in that there are not enough bipolar studies involving imaging, and among those that are available, there are very few longitudinal studies. While bipolar disorder is, in general, challenging to diagnose, the reality of high medical comorbidity and cognitive impairment in OABD may explain the dearth of longitudinal studies. In addition, longitudinal structural MRI studies, an examination of longitudinal functional MRI, and DTI studies would help to further elucidate our understanding of the neuroprogression hypothesis of BD. Although bipolar disorder is associated with significant morbidity and mortality, further research utilizing neuroimaging modalities may shed light on neurobiological mechanisms to target for therapeutic interventions designed to reduce the adverse impact of this psychiatric illness that persists into later life with often devastating consequences.
Clinical Pearls
Neuroimaging studies in OABD show gray matter volume reductions, increases in white matter hyperintensities, and neurochemical alterations.
Neuroprogression may serve as model to understand longitudinal changes in brain function associated with pathophysiology of OABD.
Better characterization of pathophysiological processes such as oxidative stress and mitochondrial dysfunction could lead to more biologically informed interventions for late-life bipolar disorder.
References
1.
Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci. 2014;8:19.CrossRefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree