Chapter 45 Coccidioidomycosis
INTRODUCTION
Coccidioidomycosis is an infection caused by the dimorphic fungus Coccidioides. The endemic region for this mycosis is confined to the Western hemisphere from California to Argentina. Areas of endemicity tend to coincide with distinct geographic and climatic conditions, principally occurring in arid, warm regions with relatively mild winters, hot summers and with alkaline soils. In the United States, the endemic region extends from the San Joaquin Valley of California, from which the common name for coccidioidomycosis, Valley Fever, is derived, to western Texas.1,2 The areas of highest endemicity are the southern San Joaquin Valley and the region encompassing Phoenix and Tucson in south-central Arizona.1–3
Recently, genetic analysis has indicated that Coccidioides can be divided into two species, C. immitis and C. posadasii.4 While it is posited that C. immitis is geographically limited to California and C. posadasii is extant in other parts of the coccidioidal endemic region, this has not been established. Moreover, there are no clinical aspects of infection that distinguish these two species. Because of this, only the genus Coccidioides will be referred to in this chapter.
Among immunocompetent individuals, coccidioidal infection is completely asymptomatic in 60% of all cases.5 The remainder usually present with nonspecific pulmonary symptoms. Approximately 1–5% of all patients develop chronic disease, either persistent pulmonary illness or infection disseminated beyond the thoracic cavity.2,6 Patients who develop chronic disease may have been asymptomatic or had symptoms at the time of initial infection.
Clinical and in vitro studies have all indicated that cell-mediated immunity is important in host defense against coccidioidomycosis.7–9 In particular, patients who have mild, self-limited disease usually manifest delayed-type hypersensitivity upon skin testing with coccidioidal antigens and have minimal serum antibody responses. On the other hand, patients with chronic, active disease, particularly those with disseminated infection, usually lack coccidioidal delayed-type hypersensitivity and have high serum anticoccidioidal antibody titers.7 Because of this, it is not surprising that coccidioidomycosis is recognized as an opportunistic infection among patients with HIV infection, particularly among those living in the coccidioidal endemic areas.10,11
EPIDEMIOLOGY
During the early part of the HIV epidemic, coccidioidomycosis was not recognized as an opportunistic process in persons infected with HIV. One reason for this was that the initial epicenters of the HIV epidemic in North America, including the Los Angeles basin, were not in areas endemic for coccidioidomycosis and reports of cases were sporadic.12–17 Another reason is that symptomatic coccidioidomycosis often occurs in individuals without any underlying immunodeficiency. The first series of cases of coccidioidomycosis occurring among HIV-infected persons was reported by Bronnimann and colleagues in 1987.18 In that report, seven of 27 patients with AIDS living in southern Arizona developed symptomatic coccidioidomycosis. Six of the seven had diffuse, nodular pulmonary infiltrates, five had detectable anticoccidioidal antibodies in their sera, and all died within 14 months of the diagnosis of coccidioidomycosis. Since that report, two large case series19,20 and one prospective study10 have been published, expanding our understanding of the clinical expression and epidemiology of coccidioidomycosis during HIV infection.
The impact of coccidioidomycosis on patients infected with HIV infection is not geographically uniform. For example, 8.2% of all patients from Arizona reported to the Centers for Disease Control (CDC) as having AIDS had concomitant coccidioidomycosis, compared to only 0.3% nation-wide.11 Moreover, 10% of all hospitalizations among HIV-infected persons in Arizona in 1993 included a discharge diagnosis of coccidioidomycosis and 44% of all hospitalizations for coccidioidomycosis were among HIV-infected patients.3
In the CDC study,11 only 0.5% of all cases of AIDS in California were reported as having concomitant coccidioidomycosis. However, the impact of HIV infection on the development of active coccidioidomycosis appears to be significantly greater in the coccidioidal endemic areas within the state. Using data from the California State Health Department, Rutherford and colleagues found that 3.5% of patients living in Kern County, a known coccidioidal endemic area, were reported as having coccidioidomycosis as their AIDS-defining diagnosis, compared to only 0.3% for the entire state.21
These data indicate that coccidioidomycosis has become a major opportunistic infection in coccidioidal endemic areas. This is illustrated by the results of a prospective study from Arizona.10 In that study, 170 HIV-infected persons who were without active coccidioidomycosis on entry were followed over time. After 41 months, 13 of these subjects developed active coccidioidomycosis, yielding an estimated cumulative incidence of ∼25%. Only two risk factors, a CD4 lymphocyte count ∼250/μL and the clinical diagnosis of AIDS, were associated with the development of active coccidioidomycosis. Length of stay in the endemic area, a history of a prior diagnosis of coccidioidomycosis, and a positive coccidioidal skin-test were not associated with a risk of developing active coccidioidomycosis. Moreover, 11 of the 13 subjects who developed active coccidioidomycosis had either focal or diffuse pulmonary involvement. All this suggests that most clinical disease was due to primary infection.
For cases in those who have not recently resided in the endemic area, reactivation of prior infection is the mechanism of disease. In some instances, reactivation may occur years after acquisition of initial infection, as in the case of a Spanish man with HIV infection who developed cervical lymphadenitis 12 years after leaving the coccidioidal endemic zone.22 Whether there are immunologic and epidemiologic differences between those who develop active coccidioidomycosis within the coccidioidal endemic area compared to those who develop it outside this area is not known. To date, coccidioidomycosis has not been reported as a major opportunistic infection among HIV-infected persons living in coccidioidal endemic regions outside the United States, such as in northern Mexico and focal areas of Central and South America.
The incidence of most infectious opportunistic infections has declined since the advent of potent antiretroviral therapy,23 and it is reasonable to presume that a decline in the incidence of coccidioidomycosis among those with HIV infection has also occurred. In a retrospective analysis of cases of coccidioidomycosis in southern Arizona from 1994 through 1997,24 a period which spans the initiation of the use of HAART, the number of cases of coccidioidomycosis among those with HIV infection declined from 77 in 1995 to 15 in 1997. While no subsequent studies have been performed, the number of cases of coccidioidomycosis among those with HIV infection seen in clinics within the endemic area appears to be lower than that observed prior to the advent of potent antiretroviral therapy. However, it is clear that coccidioidomycosis still remains a significant opportunistic infection among HIV-infected persons living within the endemic area. In one large clinic in Tucson, AZ, coccidioidomycosis was the most common cause of mortality in those with HIV infection (JK Carmichael, personal communication).
CLINICAL MANIFESTATIONS
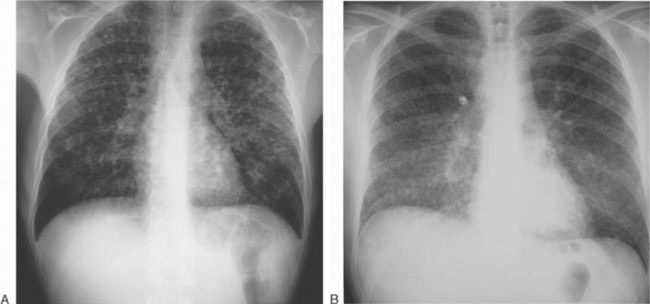
The clinical spectrum of coccidioidomycosis among HIV-infected persons has been documented in several case series reports.10,19,20 Diffuse pneumonia is the most common presentation. This is a devastating form of coccidioidomycosis, with a 70% mortality within 1 month.18–2025 It presents most often in patients with peripheral blood CD4 lymphocyte counts <50/μL, is frequently associated with fungemia, and undoubtedly represents an acute and severe form of disseminated disease. The presentation is nonspecific, with complaints of fever, night sweats, weight loss, and dyspnea. The chest radiograph reveals diffuse pulmonary nodules with increased interstitial markings, frequently described as ‘reticulonodular’. In some patients, this nodularity may be striking (Fig. 45-1A), while in others, the radiographic appearance is more subtle and suggestive of Pneumocystis pneumonia (Fig. 45-1B). Concurrent pneumocystosis occurs in up to 30% of patients with diffuse, reticulonodular coccidioidomycosis.19,26 Even with prompt antifungal therapy, this form of coccidioidomycosis is often rapidly fatal.19,20
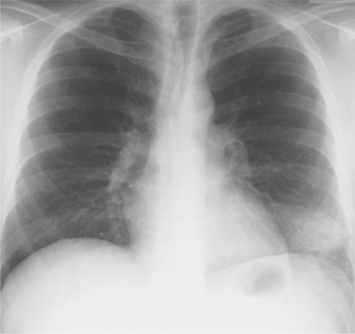
The second most frequent presentation is focal pneumonia.19,20 This presentation is more likely to occur in more immunocompetent patients with higher peripheral blood CD4 lymphocyte counts. Patients usually complain of cough, either nonproductive or productive of only scanty sputum, associated with pleuritic chest pain and fever. Bacterial pneumonia is usually the first diagnosis considered. However, symptoms persist despite antibiotic therapy. Clinical clues distinguishing coccidioidomycosis from bacterial pneumonia include the presence of mediastinal or hilar adenopathy and peripheral blood eosinophilia. The chest radiograph initially reveals a focal alveolar infiltrate (Fig. 45-2). Over time, usually weeks, this infiltrate may become nodular. The prognosis of focal pulmonary coccidioidomycosis in the HIV-infected patients is better than among those with diffuse pulmonary disease. In one study performed prior to the advent of potent antiretroviral therapy,19 the median survival of those with focal pulmonary disease was 5 months, compared to 1 month in those with diffuse pulmonary disease. Currently, the survival of HIV-infected patients with focal pulmonary coccidioidomycosis who receive appropriate antifungal and antiretroviral therapy is probably not different from patients at a similar stage of HIV infection without coccidioidomycosis.
The most frequent manifestation of extrathoracic, disseminated coccidioidomycosis in the HIV-infected patient is meningitis. Coccidioidal meningitis in patients without HIV infection usually presents with headache and a decrease in mental status occurring over a period of weeks to months, usually without any other symptoms of active coccidioidomycosis.27 The presentation is similar in the HIV-infected patient except that concurrent pulmonary involvement, particularly reticulonodular pneumonia, is more likely to be present.20 The CSF profile of HIV-infected patients is the same as in other groups of patients. There is a lymphocytic pleocytosis with hypoglycorrhachia. The finding of eosinophils in the CSF is further suggestive. Because it is uncommon to isolate Coccidioides from the CSF in cases of coccidioidal meningitis, this CSF profile may be presumed to be due to tuberculous meningitis. The diagnosis of coccidioidal meningitis should always be considered in cases of lymphocytic meningitis with hypoglycorrhachia, whether within or outside the coccidioidal endemic region.
Other manifestations of disseminated coccidioidomycosis in the HIV-infected patient include cutaneous disease, lymph node involvement, and bone and joint disease. These generally present in a manner similar to that seen in patients without HIV infection, with the exception that bone and joint disease appears to be very uncommon in HIV-infected patients. Another form of disseminated coccidioidomycosis is hepatosplenic involvement, manifesting as fever, fatigue, weight loss, and an enlarged liver and spleen in association with elevated coccidioidal serologic titers. This presentation is similar to that seen with disseminated histoplasmosis in the HIV-infected patient.28
A unique presentation of coccidioidomycosis in the HIV-infected person is a positive serum antibody reaction without clinical manifestations of illness.10,19 In one study, five of 13 individuals with positive coccidioidal serologic tests as their only manifestation of coccidioidomycosis subsequently developed active disease from 4 to 35 months after the positive serology was first noted. In four instances, patients developed pulmonary coccidioidomycosis, while in the fifth case, hepatosplenic coccidioidomycosis occurred. Prior use of antifungal therapy did not significantly reduce the risk of developing active coccidioidomycosis, but doses and duration of therapy varied considerably. The median peripheral blood CD4 lymphocyte count at the time of development of the positive serologic test was 89/μL, while it was 10/μL at the time of development of active disease. Most of the positive tests were of the complement-fixing type, with titers ranging from 1:2 to 1:128.29 These data suggest that a positive coccidioidal antibody test in an HIV-infected person represents true infection with a significant risk for the development of clinically active disease.
DIAGNOSIS
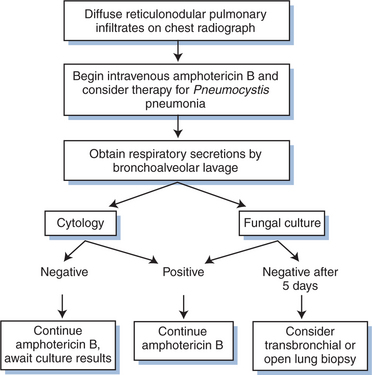
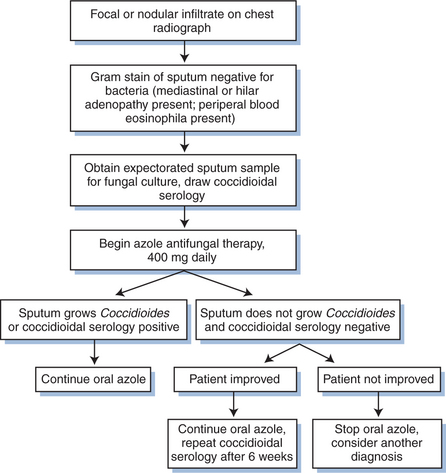
The first principle in establishing the diagnosis of coccidioidomycosis in the HIV-infected patient is to consider it. Whether within or outside the endemic area, the diagnosis should be entertained in any HIV-infected patient who presents with a diffuse pneumonia, a focal pneumonia not responsive to antibacterial agents, or a lymphocytic meningitis (see Figs 45-3 and 45-4).
Once a clinical specimen is in hand, there are several tests available to detect Coccidioides. The KOH test is commonly used on sputum and respiratory samples. While this test is simple to perform, it has a very low yield. The Papanicolaou or Gomori-methenamine stains, particularly when used on a cell pellet obtained after centrifugation of a sample obtained by bronchoalveolar lavage (BAL), have the advantage of allowing for the identification of spherules of Coccidioides as well as trophozoites and cysts of Pneumocystis.30 However, the yield of such cytologic stains for coccidioidomycosis may be as low as 40% in patients with HIV infection.31 For biopsy specimens, examination of fixed samples stained by hematoxylin-eosin usually reveals the presence of spherules. The Gomori-methenamine stain, by staining spherules and endospores black, may make the organisms more easily identifiable, but is more time-consuming to perform.
Many clinicians are under the misapprehension that Coccidioides is fastidious and difficult to cultivate. The fungus will usually display visible colonies in culture in as few as 3–7 days on a variety of culture media.6 Culture of respiratory samples and biopsy specimens has a high yield. In one study, culture of respiratory samples revealed the presence of Coccidioides in a total of 69% of cases, while lung biopsy specimens were positive in 80%.20 Because of this, any clinical specimen suspected of containing Coccidioides should be cultured.
Sequences unique to Coccidioides have been identified on the gene encoding 18S ribosomal RNA,32 making genomic identification of the fungus within clinical specimens a possibility. While genomic identification is now used to confirm the identification of Coccidioides once it is visibly growing,33 no assays are currently available for direct identification in clinical specimens. In addition, no test is clinically available to detect either antigenemia or antigenuria in coccidioidomycosis.
Without direct evidence of Coccidioides, detection of anticoccidioidal antibodies in the serum and CSF has been frequently used to establish the diagnosis of coccidioidomycosis. Understanding coccidioidal serology is complicated by antiquated nomenclature and changing methodology.34,35 Two types of antibodies are recognized. Antibodies of the IgM type, first detected using the tube precipitins (TP) method, occur early in the course of disease or during acute reactivation. Serum titers of anticoccidioidal IgM antibodies do not predict outcome and they are rarely positive in the CSF in coccidioidal meningitis. On the other hand, IgG antibodies, first detected by complement fixation (CF), rise later in the course of disease, are useful in the diagnosis of meningitis, and serum and CSF titers are helpful in following the course of the disease.
Besides the TP and CF methods, latex agglutination (LA), immunodiffusion (ID), and enzyme immunoassay (EIA) are also available for detecting anticoccidioidal antibodies. The LA test is only useful for detecting IgM antibodies and has a high false-positive rate. Any positive result should be confirmed by another method.34 The ID technique, which detects both TP (IDTP) and CF (IDCF) antibodies, is very sensitive and specific.34–36 The commercially available Premier EIA (Meridian Diagnostics, Inc, Cincinnati, OH) detects both IgM and IgG antibodies and appears to be as sensitive as ID.36–38 However false positives have been reported.36 In addition, unlike the IDCF and CF, the IgG EIA is not expressed as a titer, making it less useful in monitoring response to therapy (Pappagianis, unpublished observations).
In individuals with HIV-infection and coccidioidomycosis, tests for anticoccidioidal antibodies in the serum are positive at the time of diagnosis in 68–100% of patients.19,20,39 IgG type antibodies are more likely to be positive than the IgM types.19 While some HIV-infected patients never develop positive serologic tests for coccidioidomycosis despite active infection,39 serology remains an important diagnostic tool for most patients. It has been estimated that from 15% to 20% of patients with HIV infection and coccidioidomycosis have negative coccidioidal serologic tests, but Pappagianis has noted that only 5–7% of such samples are negative when tested in his laboratory, probably because of concentration of the serum.35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree