Fibrinogen deficient curve
% Activity
Fibrinogen, mg/dL
PT (s) (normal 10.7–15.0 s)
PTT (s) (normal 25–40 s)
100
288
11
28
80
230.4
11.4
29.8
70
201.6
11.6
29.8
40
115.2
12.6
31.9
33
95.04
13.8
34.5
30
86.4
13.1
32.6
20
57.6
14.2
34.3
10
28.8
16.6
40
5
14.4
23.4
44.5
0
0
54.1
150
12.1.4 Prothrombin Time and Partial Thromboplastin Time Mixing Studies
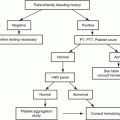
Mixing studies are used to determine if a prolongation in a PT or PTT is due to a deficiency of clotting factors, heparin or inhibitors. The test is performed by mixing patient plasma, with pooled normal plasma in a 1:1 ratio. After mixing, the PT and PTT are repeated and the times should shorten close to, or within the normal range, if the prolonged results are due to a deficiency of clotting factors. In emergency situations, mixing studies can be useful in identifying the presence of heparin as a cause of unexpected bleeding, since a lack of correction suggests the presence of heparin or an inhibitor. However, correction of the PTT does not rule out the presence of heparin in the sample (Fig. 12.1). Therefore, heparin assays should replace mixing studies for this purpose.
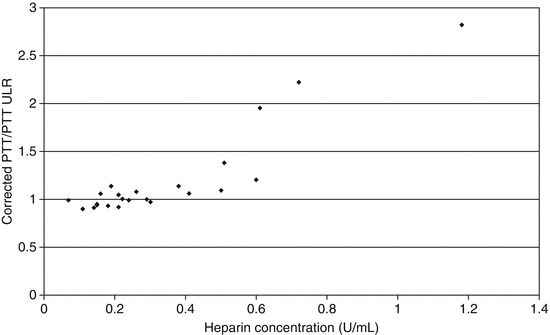

Fig. 12.1
Plot of the ratio of the corrected PTT (1:1 dilution of patient sample with pooled normal plasma) to the uncorrected PTT versus the concentration of heparin in specimens collected from patients receiving unfractionated heparin therapy. Results for corrected samples were not consistently above the upper limit of the reference interval until the heparin concentration reached 0.4 U/mL. Abbreviations: ULR upper limit of reference interval, U units
12.1.5 D-dimer Assay
D-dimer assays are very specific for the diagnosis of disseminated intravascular coagulation (DIC) [10]. The diagnosis of severe, acute DIC must be made quickly to find, and treat, the underlying cause. Early identification of DIC will also direct appropriate transfusion therapy as will be discussed later. Minor elevations in D-dimer are seen following routine surgical procedures, and in trauma patients, and should not be considered diagnostic for DIC [10–12].
12.1.6 Platelet Counts
Platelet counts are essential for identifying bleeding that may be secondary to thrombocytopenia and for directing transfusion therapy in a bleeding patient. This is an extremely valuable, but underutilized assay.
12.1.7 Thromboelastography
The search for the “Holy Grail” of coagulation assays has been ongoing for decades. The perfect assay would assess platelet number, platelet function and the soluble coagulation system with a single, rapid, inexpensive test. The thromboelastograph (TEG) is the prototype example of an attempt at such an assay. TEG has been discussed previously in this book, but in short, is a whole blood clotting assay that gives a real time graph of clot formation and stability. Standard TEG analysis was expected to be the ultimate coagulation assay because of the ability to assess all components necessary for clot formation. Subsequent studies demonstrated that the standard TEG assay was not able to differentiate between the effects of viable platelets versus cryodisrupted platelets that were added to the test system. Therefore, standard TEG analysis appears to be sensitive to the amount of phospholipid in the test system, but unable to differentiate between functional and non-functional platelets [13]. New TEG assays have been developed that use platelet agonists as initiators of clot formation in addition to the original phospholipid reagent [14]. This modification was made to better assess platelet function. These new assays have significantly increased the cost of TEG without convincing evidence of clinical benefit (see Chap. 7).
12.2 Transfusion Medicine Tools: Blood Components
12.2.1 Platelets
Platelet concentrates should be transfused in a systematic fashion. This requires consideration of three questions:
1.
Is thrombocytopenia causing life-threatening hemorrhaging?
2.
What is the cause of the thrombocytopenia?
3.
Did the patient’s platelet count increase after platelet transfusion?
The primary sites of life-threatening hemorrhage due to thrombocytopenia include the brain and the lungs. If thrombocytopenia is the suspected cause of life-threatening hemorrhage, platelets must be transfused immediately.
Bleeding patients who are not in jeopardy of death or significant morbidity should not be transfused until the cause of the low platelet count is evaluated. Understanding the etiology of the thrombocytopenia is critical because transfusion of platelets in certain disease states can actually cause morbidity and even death. Two clinical conditions where platelet transfusions should be avoided are thrombotic thrombocytopenic purpura (TTP) and heparin-induced thrombocytopenia. Platelet transfusions administered to patients with these conditions can cause an increase in platelet thrombi in tissues resulting in end organ damage.
After platelets are transfused, it is important to check a platelet count to ascertain the success of the transfusion. Many clinical conditions can lead to the immediate destruction of platelets including autoimmune thrombocytopenic purpura, sepsis, fever, splenomegaly. The administration of medications such as amphotericin and anti-thymocyte globulin can also result in platelet destruction. Patients with a prior history of pregnancy and/or transfusion are at risk for developing HLA antibodies and may need HLA matched platelets to have an adequate response to platelet transfusion.
The best way to monitor platelet transfusion success is by determining a pre-transfusion platelet count and checking the count again 1 h after completing the platelet transfusion. If time does not permit waiting for an hour (e.g. the need for an invasive procedure), a post-transfusion platelet count can be checked after 10 min. The transfusion of an adult dose of platelets (one apheresis platelet unit or six pooled, random donor platelet units derived from whole blood) should increase the platelet count by 30,000–60,000/μL. Factors determining the magnitude of the response include the number of platelets in the product, the size of the patient, and whether the patient has any clinical conditions leading to refractoriness to platelet transfusions. Calculations such as the corrected count increment or recovery rate can best quantify the success of a platelet transfusion; however, these tools are usually not necessary in routine practice.
The goal of platelet transfusion is to provide adequate circulating platelet numbers to prevent or minimize bleeding. Patients with platelet counts below 5,000/μL are generally considered to be at risk for life-threatening, spontaneous hemorrhage in the central nervous system. Prophylactic platelet transfusions for patients undergoing chemotherapy are usually given when counts are between 10,000 and 20,000/μL at the discretion of the clinician. Some clinician’s prefer to limit patient exposure to blood products and will tolerate minor mucosal bleeding and petechiae to avoid the risks of transfusion. Others transfuse platelets at counts closer to 20,000/μL to limit bleeding as much as possible.
Most patients are not at risk for severe bleeding when platelet counts are above 20,000 per μL, unless they are scheduled for an invasive procedure or have received anti-platelet medications. Most invasive procedures, or surgery, can be safely performed when counts are above 50–75,000 per/μL, but many surgeons aren’t comfortable until counts are above 100,000/μL. Striving for platelet counts significantly above 100,000/μL is neither a prudent, nor necessary use of a precious resource. Additional platelet transfusions during an invasive procedure may be necessary, and should be guided by platelet counts obtained during surgery.
The following case history demonstrates why a systematic approach to platelet transfusion is essential to optimize patient outcome. A 60 year old male was scheduled for a total hip replacement. He had a long history of aplastic anemia that required red cell transfusions. The patient’s platelet count usually ran around 30,000 per μL. He was transfused two units of apheresis platelets and taken to surgery without checking a post-transfusion platelet count. The patient required more than 70 blood product units during the procedure and almost exanguinated. Later investigation revealed the patient did not respond to the pre-operative platelet transfusions because of preformed HLA antibodies that immediately destroyed the transfused platelets.
A systematic approach to platelet transfusion could have prevented this near disaster. The patient did not have life-threatening bleeding prior to surgery, but performing a total hip replacement with a platelet count of 30,000/μL put him at risk. Prior to platelet transfusion, the cause of the patient’s thrombocytopenia should have been investigated to determine if there was a contraindication to platelet transfusion. In this case the cause was aplastic anemia and platelet transfusion was indicated. Finally, post-transfusion platelet count should have been checked to see if surgery could have been performed safely. Unfortunately, this was not done and the surgeon was not aware that the platelet transfusions had not increased the pre-operative platelet count. If the post-transfusion count had been performed, surgery would have been canceled and re-scheduled for a day when HLA matched platelets would have been available to raise the patient’s platelet count to a level adequate for a safe procedure.
12.2.2 Fresh Frozen Plasma (FFP)
Plasma is frozen soon after collection to preserve levels of the labile clotting factors V and VIII. FFP not only contains all factors in the soluble coagulation system, but also natural inhibitors of the soluble coagulation system (e.g. anti-thrombin), and proteins of the fibrinolytic system (e.g. plasminogen). FFP provides all factors necessary for the delicate balance between hemostasis and thrombosis. However, FFP is used mainly in hemorrhaging patients to replace deficiencies of multiple coagulation factors.
Hospitals are required to have laboratory guidelines for the use of FFP. The most commonly used cutoff to indicate the need for FFP transfusion is a PT and/or PTT that exceeds 1.5 times the mid-range of normal [3]. The problem with this universally applied guideline is that it does not result in a standard that can be compared between different hospitals and coagulation assay methodologies. Note that an INR of 1.5 is not necessarily equivalent to a PT of 1.5 times the mid-range of normal.
An alternative to the above calculation involves using a dilution curve to provide a guideline for FFP use (Table 12.2). A dilution curve is performed by diluting pooled, normal plasma with a known amount of crystalloid solution (e.g. normal saline) and performing PT and PTT assays on the dilutions. For example, the 50 % PT and PTT times were run on a sample of 50 % normal saline and 50 % normal plasma. The dilution curve is an in-vitro simulation of what happens when a bleeding patient is resuscitated with normal saline prior to FFP availability. The PT and PTT at the 50–40 % level are used as the guideline for FFP transfusion in the case of dilutional coagulopathy. The advantage to this approach is that it assures that the PT and PTT that are used as a guideline in a given institution fall at the same place on a dilution curve. This is not always the case when using the 1.5 times midrange of normal guideline, where the calculation may lead to a 10–20 % difference between where the PT and PTT fall on the dilution curve. While the dilution curve gives more consistency between the PT and PTT guideline values, it does not solve the problem of standardization between hospitals. For example, the 50 % cutoff will not result in the same number of seconds for PT and PTT assays in different hospitals.
Table 12.2
Sensitivity of the PT and PTT to dilution of pooled normal plasma with normal saline
Dilution sensitivity (upper limit of normal in seconds: PT = 15.5 s, PTT = 37 s) | ||
|---|---|---|
Prothrombin activity curve | ||
% Activity | PT (s) | PTT (s) |
100 | 13.6 | 29 |
90 | 14.5 | 30 |
80 | 15.2 | 31 |
70 | 15.9 | 33 |
60 | 17.2 | 36 |
50 | 19.2 | 40 |
40 | 22.4 | 48 |
30 | 27.7 | 64 |
20 | 40.3 | 99 |
15 | 52.8 | >150 |
10 | 102.8 | >150 |
5 | >150 | >150 |
1 | >150 | >150 |
Many clinicians attempt to use the INR as a guideline for FFP use. While it is true the INR will increase with a worsening coagulopathy, it has not been thoroughly evaluated as a method to direct FFP therapy. The exception would be in the setting of rapid warfarin reversal.
12.2.3 Cryoprecipitate
Historically, cryoprecipitate has been used to treat hemophilia A and von Willebrand disease, but now is used almost exclusively as a source of fibrinogen. The content, and indications for use of cryoprecipitate are not understood by many clinicians. Cryoprecipitate is produced from FFP by allowing FFP to thaw in a 1–6 °C environment. When kept at this temperature, the FFP melts, but 10–20 mL of precipitate will form. Refrigerated centrifugation separates the precipitate from the liquid. The precipitate can then be isolated, and when warmed, will go into solution. The result is a liquid, which contains fibrinogen, factor VIII, von Willebrand factor and factor XIII. Myths surrounding the use of cryoprecipitate include that it is a concentrated form of FFP and that it contains more fibrinogen than FFP. In fact, cryoprecipitate contains only the factors listed above, and only about 50 % of the fibrinogen contained in the original unit of FFP. Cryoprecipitate does not contain as much fibrinogen as FFP, but there is more fibrinogen per unit volume compared with FFP (i.e., it is a concentrated source of fibrinogen). Each bag of cryoprecipitate contains, on average, about 200 mg of fibrinogen. As noted earlier in this chapter, fibrinogen levels of 80–100 mg/dL are required to achieve hemostasis in a bleeding patient. To raise the fibrinogen level in an adult by 100 mg/dL requires between 10 and 30 bags of cryoprecipitate depending on the size of the patient (about two bags per 10 kg of body weight). The most common error in cryoprecipitate transfusion is delivery of a dose insufficient to significantly increase fibrinogen levels in an adult (e.g. four bags). Patient fibrinogen levels should always be re-evaluated after cryoprecipitate infusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree