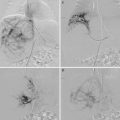
Fig. 4.1
Coagulation cascade and initiation of LIC (coagulopathy) and KMP (platelet trapping). KMP kasabach merrit phenomenom, FDP fibrin degradation product, KHE kaposiform hemangioendothelioma, LIC localized intravascular coagulopathy, TA tufted angioma
The coagulation process involves successive protease reactions leading to the conversion of soluble fibrinogen into insoluble fibrin. Fibrin strands linked to aggregated platelets form a stable thrombus. The new cell-based model of coagulation explains the mechanism of hemostasis in vivo. This process is initiated when TF-bearing cells are exposed to blood at the site of endothelial injury or abnormal endothelium (vascular abnormalities). Briefly, the initiation of the coagulation leads to the production of small amounts of thrombin (FIIa) that amplify its own generation (amplification phase). The burst of thrombin induces the formation of cross-linked fibrin (FIa). Similarly, platelet activation on altered endothelium leads to platelet aggregation and subsequently to the formation of FIIa and fibrin clots. Fibrinolysis is essential for clot dissolution. Fibrin plug is cleaved by plasmin with the production of fibrin degradation products (FDP) and D-dimers.
KMP and Vascular Tumors
Introduction
In 1940, Kasabach and Merritt described an infant with a “capillary hemangioma” with extensive purpura and profound thrombocytopenia [1]. This type of coagulopathy was named Kasabach–Merritt phenomenon (KMP) and was assumed to be a complication of common infantile hemangioma. In 1980 and 1993, kaposiform hemangioendothelioma (KHE) and tufted angioma (TA) were differentiated from infantile hemangioma (Part II Chap. 7) [2–4]. Review of different cases presenting with KMP revealed that these children did not have classical infantile hemangioma but KHE or TA [5, 6]. TA and KHE are now considered as benign vascular tumors with similar presenting symptoms with a potential for developing KMP. Histologic examination usually can distinguish between KHE and TA but overlap of histologic characteristics can be observed especially on small biopsy specimens. KMP can be also associated with malignant vascular tumors such as angiosarcoma [7, 8].
Epidemiology and Predisposing Factors
Kasabach–Merritt phenomenon is very rare, occurring in 0.3 % of benign vascular tumors but in more than 50 % of the cases of kaposiform hemangioendothelioma and tufted angioma during the first year of life [9]. These tumors are either congenital or appear postnatally. There is no sex ratio and no familial cases or underlying genetic mutations. To date, in the largest cohort of 107 KHE patients, KMP developed in 71 % of the patients, and the greater risk factors were mediastinal or retroperitoneal involvement, deep infiltration of the tumor into muscle or fascia, and large cutaneous lesions (>5–8 cm) [10, 11]. Enjolras et al. found that histologic features of KHE were more common during the active phase of KMP compared with features of TA in the beginning of KMP or in the residual after cure [12]. In this chapter, we shall use KHE/TA as an all-encompassing term.
Clinical Features
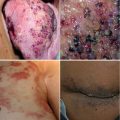
KHE/TA is usually solitary tumor which develops on the lateral neck, axilla, groin, extremities, and trunk. When associated with KMP, it appears as an ecchymotic mass with centrifugal purpura. It can also occur in noncutaneous and sometimes hidden locations: retroperitoneum, mediastinum, or bones. Therefore, thrombocytopenia in a newborn unexplained by other routine tests (such as for sepsis) should prompt for occult vascular tumor. Multifocal lesions have rarely been reported [13, 14].
Laboratory Features and Pathogenesis (Fig. 4.1)
KMP is characterized by severe thrombocytopenia (<20 × 109/L) associated with hypofibrinogenemia, increase of fibrin degradation products (PDF) and presence of schizocytes due to red cell fragmentation by fibrin filaments. Anemia is not a common presenting sign unless significant bleeding occurs within the tumor. As a consequence of platelet activation, platelet half-life is drastically shortened to 1–24 h.
KMP belongs to a spectrum of diseases characterized by a thrombotic microangiopathy (TMA). TMA results from red cell activation and platelet trapping with subsequent thrombocytopenia and microangiopathic hemolytic anemia with negative Coombs test [9]. Although the pathogenesis of this syndrome is not well elucidated, it is probable that the abnormal endothelium of the associated vascular tumors (KHE/TA) leads to platelet trapping and aggregation followed by activation of coagulation factors, fibrin deposition, and subsequently red blood cell damage and consumptive coagulopathy (DIC). Fibrinolysis occurs with consumption of clotting factors and intralesional bleeding with rapid enlargement of the tumor.
In addition, liver immaturity of the premature neonates may enhance impairment of coagulation because of low synthesis of clotting factors [15].
Treatment
Treatment is challenging without a consensus on the optimal medical management. There are no standardized protocols, no prospective studies, and no long-term follow-up published in the literature [16]. The goal of the treatment of KMP is to reduce the tumor size as it generates hematologic instability. In 2013, a multidisciplinary and multi-institutional expert panel published a standardized consensus-derived standard of practice for the treatment of KHE/TA with and without KMP [17]. Medical therapy is the first approach as KHE/TA with KMP is usually deep and infiltrative, and surgical excision is considered when the tumor can be safely and completely excised. There is a consensus on the fact that KHE/TA should be treated aggressively with a combination regimen administered as soon as possible. The first-line recommended pharmacologic protocol is intravenous vincristine (0.05 mg/kg) once weekly AND oral prednisolone 2 mg/kg/day OR intravenous methylprednisolone 1.6 mg/kg/day. However, if administration of vincristine is not immediately possible (lack of central venous access), treatment must not be delayed, and corticosteroid therapy should be started before the availability of vincristine. The duration of the treatment depends on the individual clinical response and stabilization of the coagulopathy. Corticosteroids can be weaned after 3–4 weeks of full-dose treatment. The average length of vincristine therapy is usually 20–24 weeks, but it depends on the shrinkage of the tumor, the stabilization of the hematologic status, and the toxicity of the drug. Complete clearance of the tumor is rare; residual fibrotic lesions can be visualized by follow-up imaging, and there is no point in prolonging therapy although these residua are considered as dormant vascular tumors and not scars [12]. Interferon alpha has been used with success but was associated with a risk of spastic diplegia in infants less than 8 months of age [18]. The use of propranolol cannot be recommended because varied responses to treatment have been observed [19, 20]. Sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, is widely used in patients following organ transplantation. It also has an antiangiogenic activity and may have an application in the treatment of vascular lesions. Rapid improvement of hematologic disorders with no recurrence several years after treatment was reported in two patients with KHE/TA associated with KMP [21, 22]. It seems to be a promising treatment, and prospective studies are needed to evaluate safety and efficacy of this agent.
Adjuvant therapies are used to improve thrombocytopenia and diminish the risk of bleeding. Platelet transfusion increases platelet trapping and may induce enlargement of the tumor and increased pain. Moreover, local platelet activation leads to the release of proangiogenic factors and possible tumor proliferation [23]. Therefore, platelet transfusions are only indicated for active bleeding and/or immediately prior surgery. Inhibitors of platelets’ function such as aspirin, ticlopidine, and clopidogrel have been used in combination with other therapies to prevent platelet activation. They are not effective in preventing platelet consumption, but aspirin may be successful in controlling pain [24, 25]. The use of antifibrinolytic agents such as ε-aminocaproic acid or tranexamic acid was proposed because it is considered that inhibition of fibrinolysis might delay fibrin lysis and reduce bleeding. However, in the published cases, when important bleeding occurred, these agents rarely reduced blood transfusions [26, 27]. When bleeding is associated with hypofibrinogenemia (<100 mg/dl), administration of fibrinogen or fresh frozen plasma is recommended to replace the consumed clotting factors. Heparin is not indicated because of absence of effect on platelets and bleeding risk.
Multifocal Lymphangioendotheliomatosis with Thrombocytopenia (MLT)
Introduction
Multifocal vascular lesions have recently been classified as multifocal lymphangioendotheliomatosis with thrombocytopenia (MLT) and multifocal infantile hemangiomatosis (IH) [28]. MLT has a much higher mortality compared to IH because of severe gastrointestinal (GI) bleeding.
Clinical and Histologic Features
Skin lesions in MLT range from 1 to 2 mm telangiectatic plaques to large exophytic hemorrhagic nodules. They are associated with similar GI lesions distributed diffusely throughout the GI tract. GI bleeding in MLT is often profuse causing significant morbidity or even death. Multifocal IH is clinically different as cutaneous lesions are small disseminated red papules which may occasionally be associated with segmental GI involvement, but the main extracutaneous site is the liver. MLT and IH have similar histopathologic findings with dilated vessels in the dermis and subcutis. To differentiate MLT from infantile hemangioma, relevant stains are necessary to confirm the absence of the specific marker of infantile hemangioma GLUT 1 and the positivity of a lymphatic endothelial cell-specific marker LYVE-1 (lymphatic endothelial receptor-1) or podoplanin (D2–40) on the lining of the dilated vessels. MLT’s lesions co-express blood and lymphatic markers, like kaposiform hemangioendothelioma and angiosarcoma endothelial cells. Lesions are slowly progressive in the first years of life and remain stable, contrary to multifocal IH which are rapidly progressive in the first months of life and disappear within 1–5 years.
Laboratory Findings and Pathogenesis
Mild thrombocytopenia is reportedly associated with anemia due to GI bleeding. A consumptive coagulopathy is observed with elevated D-dimer levels.
Pathogenesis of MLT is unknown, but thrombocytopenia is probably also due to platelet destruction within the vascular lesions which have indeterminate blood/lymphatic vascular endothelial differentiation [29].
Treatment
Contrary to IH, MLT does not respond to usual treatments such as prednisone, propranolol, vincristine, and interferon. Bevacizumab, an antibody to the vascular endothelial factor, has been used with success in one case [30].
Localized Intravascular Coagulopathy (LIC) and Venous Malformations (VMs)
Clinical and Laboratory Features
Venous malformations (VMs) are associated with spontaneous thrombosis and thrombolysis. This is witnessed by elevated D-dimer levels in 42 % of patients and associated with size, deepness, and presence of palpable phleboliths [31–33]. This phenomenon is named localized intravascular coagulopathy (LIC). D-dimer levels are often very high (25 % of patients >1.0 mg/mL), even if these otherwise healthy patients do not have other conditions to increase D-dimer levels, e.g., cancer, inflammatory disease, thrombophilia, ischemic heart disease, arterial aneurysm or dissection, or pregnancy. In other conditions, such as oral contraception, ulceration, and old age, D-dimer levels can also mildly increase, but levels are much lower than that reported for VMs [33–35]. VM is the only disease that can permanently highly increase D-dimer levels in otherwise healthy patients. Thus, it is a biomarker helpful for diagnosis. When D-dimer levels are elevated in vascular anomaly patients with no associated pathology, a venous component is present in 96.5 % of patients. This is true for pure, isolated VMs (uni- or multifocal) as well as for combined and syndromic lesions (e.g., capillary venous malformation and Klippel–Trenaunay syndrome (KTS)) (Chaps. 22 and 23). In contrast, sensitivity is lower (42 %). Thus, when D-dimer levels are normal, a small VM cannot be ruled out.
Among all patients with vascular malformations, D-dimer levels are normal in all glomuvenous malformations (GVMs), lymphatic malformations (LMs), and Maffucci syndrome as well as in fast flow malformations such as arteriovenous malformations (AVMs) and Parkes Weber syndrome (Chaps. 22 and 23). Thus, D-dimer measurement is a useful biomarker for the differential diagnosis of VMs. It can help, e.g., in differentiating GVMs from other multifocal venous lesions. It can also detect a venous component in combined and syndromic malformations. Thus, this easy and cheap biomarker test must be used as a routine test in clinical evaluation of vascular anomaly patients [36].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree