Clinical Evaluation and Management of Thyroid Nodules
Susan J. Mandel
Jill E. Langer
Michael M. Kaplan
Thyroid nodules are relatively common in adults. On physical examination of the neck, a thyroid nodule is considered to be present when a portion of the thyroid has a different contour or consistency than the rest of the gland. On sonography, a nodule is defined as a focal region of differing parenchymal echotexture (Table 49.1). Combined data from several studies in which ultrasonography was used as the method of nodule detection reveal a roughly linear increase in the prevalence of thyroid nodules from near zero at the age of 15 years, to 50% by about age 60 to 65 years, and to even higher in older people (1). At most, 10% of these nodules are palpable, even by experienced examiners (1,2). The dilemma for the physician is to identify the few patients who have thyroid nodules that pose a health risk from the vast majority in whom the nodules pose no harm. Optimally, the selection process will have high diagnostic accuracy, minimize medical costs and patient’s anxiety and lead to treatment that will improve the patient’s health.
Table 49.1 Types of Thyroid Nodules | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Although a key consideration in thyroid nodule evaluation is whether the nodule is a thyroid carcinoma, many thyroid cancers may be indolent. At autopsy, up to 30% of thyroid glands harbor malignant nodules under 1 cm, termed microcarcinomas by the World Health Organization (3). Most of these microcarcinomas lack the potential to become biologically significant and have an indolent course. However a small
percentage of these subcentimeter cancers do exhibit aggressive behavior and may be invasive and metastasize. Overall, evaluation of thyroid nodules found either on physical examination or by imaging studies reveals that between 7% and 9% are differentiated thyroid cancers of follicular-cell origin (4). The ultimate goal is to diagnose biologically significant cancers, while exposing the fewest number of patients with benign disease or indolent malignant disease to unnecessary diagnostic testing and thyroid surgery. In addition, some thyroid nodules, although benign, require treatment because either they cause thyrotoxicosis or their size causes an unacceptable appearance or local symptoms with compression of nearby structures in the neck and upper chest.
percentage of these subcentimeter cancers do exhibit aggressive behavior and may be invasive and metastasize. Overall, evaluation of thyroid nodules found either on physical examination or by imaging studies reveals that between 7% and 9% are differentiated thyroid cancers of follicular-cell origin (4). The ultimate goal is to diagnose biologically significant cancers, while exposing the fewest number of patients with benign disease or indolent malignant disease to unnecessary diagnostic testing and thyroid surgery. In addition, some thyroid nodules, although benign, require treatment because either they cause thyrotoxicosis or their size causes an unacceptable appearance or local symptoms with compression of nearby structures in the neck and upper chest.
Clinical Evaluation
Most patients with thyroid carcinoma present with an asymptomatic thyroid nodule, as do most patients with benign thyroid nodules, and the most common method of detection appears to have shifted from physical examination to incidental imaging by radiology studies. Recently published evidence-based guidelines by professional societies, the American Thyroid Association (ATA) and a consortium of the American Association of Clinical Endocrinologists, the Asscociazione Medici Endocrinologi and the European Thyroid Association (AACE/AME/ETA), provide similar recommendations for the evaluation and management of patients with thyroid nodules (5,6), beginning with history and physical examination and then progressing to diagnostic testing and therapeutic recommendations. Although the available literature linking clinical variables with cancer risk is summarized in the discussion below, it is important to consider the effect of potential selection bias in these studies, since many predate the widespread use of ultrasound and therefore do not include small and incidentally found thyroid nodules.
Table 49.2 Clinical Findings Related to Risk of Carcinoma in A Thyroid Nodule | |
|---|---|
|
A thyroid nodule is more likely to be a thyroid carcinoma in patients under 20 years of age and those over 65 years of age than in those in between, but overall most patients with thyroid carcinoma are in this middle age group (Table 49.2). Benign thyroid nodules are four to five times more common in women than men, but thyroid carcinomas are only two to three times more common in women; thus, a nodule is more likely to be a carcinoma in a man (7). Family history is relevant. Although the genetics are not yet delineated, 4% to 8% of patients with papillary thyroid carcinoma have a family history of the same tumor (8). In familial adenomatous polyposis and its variant, in Gardner’s syndrome, and in Cowden’s disease, also called multiple hamartoma syndrome, the prevalence of benign thyroid nodules and thyroid carcinoma is increased (8) (see section Pathogenesis in Chapter 50B). Medullary thyroid carcinoma can be familial (see Chapter 51), as part of the Multiple Endocrine Neoplasia type 2 and related syndromes, in which there is a very high penetrance of medullary carcinoma, and less often pheochromocytoma and primary hyperparathyroidism.
Radiation therapy directed to the head, neck, and upper chest increases the risk of both benign thyroid nodules and thyroid carcinoma (see section on pathogenesis in Chapter 50A). In the past, most patients with radiation-related thyroid carcinoma had received radiation therapy for benign conditions, such as thymic or tonsillar enlargement, acne, or birthmarks in infancy, childhood, or adolescence, but use of this therapy ceased in the early 1960s. Current therapeutic radiation-related thyroid carcinoma typically occurs in patients who as children up to the age of 18 received treatment for malignancies that involved radiation to the head, neck or upper mediastinum such as for Hodgkin’s disease, preventive brain irradiation as in acute leukemias or total body irradiation prior to bone marrow transplant. These predominantly papillary cancers are reported to occur a median of 13 to 15 years after initial therapy, but thyroid cancers can be diagnosed up to three decades after radiation exposure, emphasizing the need for a long-term surveillance strategy (9,10). In addition, fallout from the 1986 Chernobyl nuclear power plant explosion dramatically increased
the incidence of thyroid cancer in exposed children, who are now 25 to 35 years old (11).
the incidence of thyroid cancer in exposed children, who are now 25 to 35 years old (11).
Sudden growth of a nodule, often accompanied by pain, usually indicates hemorrhage into a cystic nodule, which occurs in both benign and malignant nodules. In addition, subacute thyroiditis may present with unilateral thyroid enlargement and pain, mimicking a thyroid nodule. Although anaplastic thyroid cancer and thyroid lymphoma exhibit rapid growth, these tumors are exceedingly rare. Large thyroid nodules, benign or malignant, can distort surrounding structures in the neck or upper mediastinum, causing difficulty breathing, shortness of breath or cough from tracheal compression, or difficulty swallowing from esophageal compression. The presence of these symptoms may increase the likelihood that a nodule is malignant, but most carcinomas are asymptomatic. Similarly, hoarseness or a change in voice can be caused by infiltration or compression of the recurrent laryngeal nerve by a thyroid carcinoma, but other causes of a change in voice are much more common. The presence of non-tender cervical adenopathy also raises concern for malignancy. Whether nodule size itself is a risk for thyroid malignancy is controversial. Although some studies have reported size is correlated with cancer (12,13), others have concluded that it is not an independent factor for predicting malignancy (14,15).
Thyroid nodules incidentally detected on computed tomography (CT), magnetic resonance imaging (MRI), or on carotid artery sonography are best assessed for features of malignancy by dedicated thyroid sonography, similar to nodules detected by palpation. However, thyroid nodules incidentally identified by 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) have a higher likelihood of being malignant (∼30%), but the majority are still benign (16). Hashimoto’s thyroiditis takes up substantial quantities of 18-fluorodeoxyglucose that may be asymmetric, mimicking a discrete lesion (17). Therefore, subsequent sonographic assessment determines if the FDG uptake corresponds to a nodule, which then requires fine-needle aspiration (FNA), or to the diffusely heterogeneous thyroid parenchyma of lymphocytic thyroiditis without a superimposed discrete nodule. In addition, nodule uptake of technetium-99m methoxy isobutylisonitrate (MIBI), used for myocardial and parathyroid imaging, carries a higher risk of malignancy in some studies (18).
Up until the mid-1990s, it was widely believed that the risk of thyroid malignancy was higher in patients with solitary nodules as compared with multinodular glands. More recent published series that define gland nodularity by imaging document that a patient’s risk of malignancy is independent of the number of nodules, as well as whether the nodule is palpable (19,20). Therefore, the distinction between solitary thyroid nodules and multiple nodules is now recognized to be spurious in most patients, because over 50% of patients, thought on the basis of physical examination to have a solitary nodule, have multiple nodules when studied by ultrasonography (2,20).
Laboratory Evaluation
After obtaining a history and performing a physical examination in patients with thyroid nodules, a serum thyrotropin (TSH) should be obtained because this result will lead to two different initial imaging pathways, either thyroid ultrasound (US) or radionuclide imaging. Even if thyroid scintigraphy is indicated as the initial imaging modality (see Radionuclide Imaging below), a recent study demonstrated that the combination of I-123 scanning with sonography is still recommended for assessment of FNA necessity in patients with functioning nodules because 10% of non-functioning nodules meeting FNA criteria would be missed if the sonogram was not performed (21).
Serum Hormone and Other Measurements
Serum TSH should be measured to determine if the patient potentially has an autonomously functioning thyroid adenoma causing thyrotoxicosis. FNA for cytology determination is not indicated in functioning nodules because of the extremely low malignancy risk, <5%; however such patients require evaluation and possible treatment for hyperthyroidism. Several recent studies have demonstrated that even within the normal range, higher serum TSH levels are an independent risk factor for malignancy in patients with thyroid nodules (7,14). Therefore, TSH measurement confers an additional risk level to a thyroid nodule and may lead the clinician toward FNA at the lower size cutoff within a recommended range, e.g., a sold isoechoic nodule where ATA guidelines recommend FNA for nodules larger than 1 to 1.5 cm (5).
Measurement of serum thyroglobulin has little value in patients with thyroid nodules; the values may be normal or high in patients with benign nodules, Hashimoto’s thyroiditis, or carcinoma. Measurement of serum calcitonin, as well as testing for germline RET proto-oncogene mutations, is indicated in patients with a nodule if there is a family history of medullary thyroid cancer (MTC) or other components of the Multiple Endocrine Neoplastic type 2 syndrome. Several recent European studies have analyzed the utility of routine serum calcitonin measurement in patients with thyroid nodules for identification of MTC (22,23). Although mild elevations of serum calcitonin >10 pg/mL are highly sensitive for MTC detection, these are not specific (24) and pentagastrin stimulation is required to exclude false-positive results. Furthermore, most of the medullary cancers in these studies were correctly identified by FNA cytology results, and the cost-effectiveness of routine calcitonin measurement in the United States is being debated (25). The lack of availability of pentagastrin in the United States limits applicability of screening calcitonin measurements.
Imaging Studies
As noted above, the serum TSH result determines the appropriate next imaging study for thyroid nodule evaluation.
Table 49.3 Grayscale Sonographic Features Reported to Be Associated with Thyroid Cancer (27) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Ultrasonography
If the serum TSH level is normal or high, thyroid ultrasonography (US) should be performed in all patients with palpable thyroid abnormalities and in those with suspected thyroid nodules detected on other radiology studies. For patients with palpable thyroid nodules, thyroid US may document either that the palpable abnormality is not a discrete nodule, or that there are additional nodules that warrant evaluation based on size
and appearance (2,20). In addition, the sonographic features of a nodule confer a degree of risk for thyroid cancer, and may potentially exclude the possibility of cancer (26,27). Ultrasound imaging also demonstrates the anatomic location of the nodule in the thyroid (anterior/posterior) as well as the composition (cystic/solid), factors important in determining whether an FNA could be performed by palpation, or whether sonographic guidance is required. Lastly, ultrasound is more accurate for nodule size assessment than palpation and provides the size of the nodule in three dimensions, which is required for follow up.
and appearance (2,20). In addition, the sonographic features of a nodule confer a degree of risk for thyroid cancer, and may potentially exclude the possibility of cancer (26,27). Ultrasound imaging also demonstrates the anatomic location of the nodule in the thyroid (anterior/posterior) as well as the composition (cystic/solid), factors important in determining whether an FNA could be performed by palpation, or whether sonographic guidance is required. Lastly, ultrasound is more accurate for nodule size assessment than palpation and provides the size of the nodule in three dimensions, which is required for follow up.
Even after acknowledging methodologic differences and interobserver variation, studies have consistently reported the association of certain sonographic features with thyroid malignancy (27) (Table 49.3). These include hypoechogenicity, particularly marked hypoechogenicity, solid composition, infiltrative or microlobulated margins, presence of calcifications (especially microcalcifications), and taller than wide shape when measured in the transverse view.
Nodule hypoechogenicity (appearing darker than the normal thyroid parenchyma) is thought to arise from the increased cellularity and cellular compaction that is present in the classic papillary and MTCs. In fact, a markedly hypoechoic nodule, which images as darkly as the very hypoechoic strap muscles surrounding the thyroid, is highly predictive of thyroid cancer (28,29). In contrast, a follicular adenoma or carcinoma, as well as a follicular variant of papillary cancer, are composed of microfollicles with colloid that provide reflecting surfaces, resulting in a more iso- or hyperechoic appearance. Thyroid cancers of all histologies are most commonly predominantly solid. In fact, sonographic evaluation of 360 thyroid cancers removed at the Mayo clinic demonstrated that only 9.2% were 6% to 50% cystic and even fewer, 2.9%, were 51% to 100% cystic. This small proportion of cystic thyroid malignancies generally demonstrates other suspicious ultrasound features in the solid component, such as a mural solid component, microcalcifications, increased vascularity or an irregular border (30). Sonography performed with high frequency, high resolution transducers allows detailed assessment of the interface of thyroid nodules with the surrounding parenchyma. The detection of infiltrative, speculated margins or a microlobular border is therefore concerning for an unencapsulated invasive thyroid carcinoma. However, the distinction between infiltrative borders and indistinct borders is important because many small hyperplastic nodules will have poorly defined margins separating hyperplastic from normal tissue, but these are not infiltrative (28). The presence of any type of calcification within a nodule [microcalcification, dense (also called macrocalcification), or curvilinear] increases the likelihood of cancer (4), with microcalcifications having the highest specificity for papillary thyroid cancer. Disproportionate growth in the anterioposterior dimension (taller than wide shape) is considered an aggressive growth pattern across, rather than within the normal tissue planes, and has been associated with malignancy, especially in smaller cancers. Although increased intranodular vascularity has been reported by earlier studies to be a worrisome feature, a recent report analyzing over 1000 nodules did not confirm its independent association with papillary thyroid cancer, after accounting for gray scale features, and, in fact, lack of vascularity was frequently observed in hypoechoic cancers (29).
One reason for the lack of high sensitivity for these features is that different thyroid cancer histologies image differently with sonography. For example, a classic papillary thyroid cancer is more likely to be hypoechoic with infiltrative margins and microcalcifications than either the follicular variant of papillary cancer or follicular cancer, which are more often iso- to hyperechoic with regular margins and lack microcalcifications (31,32). With the exception of the presence of metastatic cervical lymphadenopathy, no feature or combination of sonographic features is sufficiently sensitive to be the sole screening test for thyroid cancer.
Conversely, the absence of suspicious sonographic features may identify nodules that are benign. Two such appearances are highly correlated with benignity, a spongiform nodule and a pure cyst. A nodule is categorized as spongiform when more than 50% of nodule volume is occupied by microcystic spaces; this appearance has a 98.5% negative predictive value for malignancy (28,33). Nodules which are entirely composed of cystic fluid are relatively rare (<2% of all nodules), but are virtually always benign. Often, comet tail artifact, representing reverberation of sound waves caused by inspissated colloid, is present in these pure cysts.
On the basis of the sonographic imaging features, nodule appearance can be classified as low, intermediate, and high risk for malignancy (Fig. 49.1) (34). Low risk nodules are pure cysts or spongiform, remembering that small spongiform nodules may have indistinct, but non-infiltrative borders. In addition, predominantly cystic nodules with a flat peripheral solid
component or a spongiform solid area are also usually benign (34). Solid or predominantly solid, hypo- or iso- to hyperechoic non-calcified nodules with regular margins are intermediate risk as are nodules that have a defined solid area within a complex nodule. Hypoechoic nodules with microcalcifications and infiltrative or microlobulated margins are high risk, as are any nodules, including cystic nodules, with true microcalcifications. Both ATA and AACE/AME/ETA guidelines recognize pure cystic and spongiform nodules as low risk for malignancy and the size cutoffs for FNA of such lesions are more lenient (if performed at all) (see FNA section below) than for intermediate or high risk nodules (5,6).
component or a spongiform solid area are also usually benign (34). Solid or predominantly solid, hypo- or iso- to hyperechoic non-calcified nodules with regular margins are intermediate risk as are nodules that have a defined solid area within a complex nodule. Hypoechoic nodules with microcalcifications and infiltrative or microlobulated margins are high risk, as are any nodules, including cystic nodules, with true microcalcifications. Both ATA and AACE/AME/ETA guidelines recognize pure cystic and spongiform nodules as low risk for malignancy and the size cutoffs for FNA of such lesions are more lenient (if performed at all) (see FNA section below) than for intermediate or high risk nodules (5,6).
Radionuclide Imaging
Prior to the use of ultrasound, thyroid scintigraphy was usually the first imaging study performed in patients with thyroid nodules because nodules identified as autonomously functioning (concentrating the radionuclide more than the rest of the thyroid gland) are rarely malignant, and FNA evaluation is not indicated. However, only about 5% to 10% of nodules are autonomously functioning (Fig. 49.2), nearly all of which are adenomas (35). In some of these tumors, activating mutations of the TSH receptor (36) or the guanine nucleotide-binding (G)
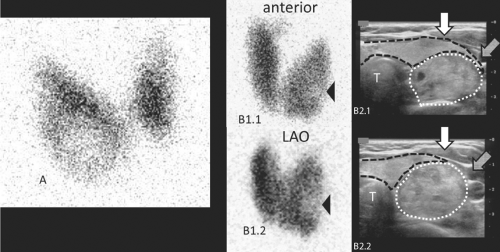
protein that links the TSH receptor with adenylyl cyclase (37) cause the TSH-independent function. Since more than 90% of nodules do not produce thyroid hormone (“non-functioning”) (Fig. 49.3), it is not cost-effective to perform radionuclide imaging as a screening test for all patients with thyroid nodules. Rather, scintigraphy, with either 123I or 99mTc pertechnetate, is limited to those patients who have subnormal serum TSH levels (5,6), where the diagnostic possibilities include: Functioning nodule(s), a combination of functioning and non-functioning nodules, or Graves’ disease with non-functioning nodules.
protein that links the TSH receptor with adenylyl cyclase (37) cause the TSH-independent function. Since more than 90% of nodules do not produce thyroid hormone (“non-functioning”) (Fig. 49.3), it is not cost-effective to perform radionuclide imaging as a screening test for all patients with thyroid nodules. Rather, scintigraphy, with either 123I or 99mTc pertechnetate, is limited to those patients who have subnormal serum TSH levels (5,6), where the diagnostic possibilities include: Functioning nodule(s), a combination of functioning and non-functioning nodules, or Graves’ disease with non-functioning nodules.
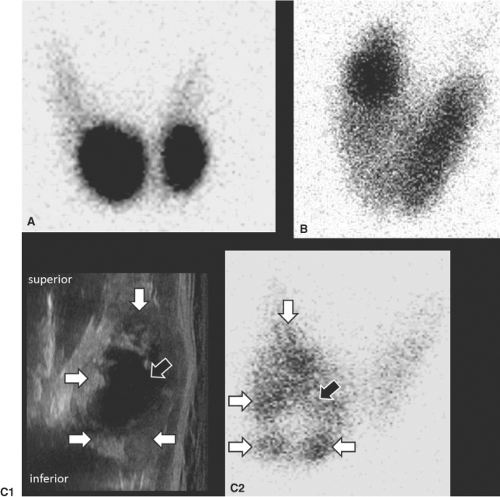
Many functioning nodules may be composed of both solid and cystic areas and direct correlation of the two exams may be necessary. Cystic components of functioning nodules do not produce thyroid hormone and ultrasound imaging is required to confirm that scintigraphically non-functioning areas of an otherwise hyperfunctioning nodule correspond to cystic areas (Fig. 49.2C1, 49.2C2) (21). If not, FNA is required to target a solid non-functioning area. In addition, both functioning and non-functioning nodules may be present within a gland, and sonographic correlation may be necessary to identify non-functioning nodules within suppressed extranodular parenchyma, for which FNA is indicated on the basis of sonographic features. Lastly, in both euthyroid patients and those with Graves’ disease, smaller nodules (<2 cm) may not appear as non-functioning on planar scintigraphy because of the surrounding functioning thyroid parenchyma. Sonography will identify these nodules and determine if FNA is indicated (21,27,38) (Fig. 49.3B).
Table 49.4 American Thyroid Association Recommendations for Thyroid Nodule Fna (5) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
123I scintigraphy may have a role after FNA evaluation in a subset of nodules with follicular neoplasm cytology to assess autonomy if the serum TSH concentration is in the low normal range (5,6). Some autonomously functioning adenomas have this cytology (17,39), but do not secrete enough thyroid hormone to lower TSH secretion, especially if less than 2.5 cm in diameter (35), and yet are active enough to be revealed as hyperfunctioning by scintiscan (Fig. 49.2B). The risk for carcinoma is low in this subset of patients, just as it is in those patients with adenoma who have low serum TSH concentrations.
Other Imaging Studies
Computed tomography (CT), MRI, and 18FDG-PET have no established role in the initial evaluation of patients with thyroid nodules. Cross-sectional imaging may be helpful for pre-operative planning after diagnosis of thyroid cancer, if there is concern about local invasion into surrounding structures, such as the esophagus and trachea. It may also be useful in patients with benign thyroid nodules who complain of compressive symptoms.
Fine-Needle Aspiration Biopsy
FNA biopsy, which yields a cytology specimen for analysis, is the standard test to determine whether surgical removal is recommended. The general term FNA will be used here; however, aspiration by negative pressure is not always employed (see later). The primary goal of FNA is to reduce the number of unnecessary thyroid operations, by identifying those nodules that are benign or at least unlikely to be malignant. For this purpose, fine-needle biopsy is far more accurate than any other available test or combination of tests. Before the procedure was introduced, about half of the patients with palpable thyroid nodules were referred for operation, but only about one-fourth of those who underwent surgery had thyroid carcinoma. Hence, many patients with benign nodules were subjected to the costs, morbidity, and, rarely, risk for mortality of surgery (40).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree