Chronic Rhinosinusitis Role of Rhinoscopy and Surgery
Rakesh K. Chandra
David B. Conley
Robert C. Kern
Introduction and Historical Perspective
Chronic rhinosinusitis (CRS) affects an estimated 31 million people in the United States. Management of this disorder, which accounts for approximately 16 million patient visits per year, has changed dramatically in the past 50 years. This is due to new insights into the pathophysiology of sinusitis, advances in rhinoscopy (nasal endoscopy), improved radiographic imaging, and availability of antibiotics (1). Technical advances in endoscopic instrumentation have defined a new era in the office diagnosis and surgical management of sinusitis, permitting an unprecedented level of precision. Understanding the indications as well as the technical limitations of diagnostic and therapeutic rhinoscopy is now essential for practitioners who manage CRS.
Hirschman performed the first fiberoptic nasal examination using a modified cystoscope (2). Refinements in instrumentation after World War II allowed the development of smaller scopes that provided better illumination. In the early 1950s, investigators at Johns Hopkins University designed a series of endoscopes with relatively small-diameter, wide-field, high-contrast optics, and adequately bright illumination. At this time W. Messerklinger of Graz Austria began to use this technology for systematic nasal airway evaluation. He reported that primary inflammatory processes in the lateral nasal wall, particularly in the middle meatus, result in secondary disease in the maxillary and frontal sinuses (2). This region, which represents a common drainage site for the maxillary, frontal, and anterior ethmoid sinuses, is termed the osteomeatal complex (OMC). Messerklinger found that small anatomic variations or even minimal inflammatory activity in this area could result in significant disease of the adjacent sinuses as a result of impaired ventilation and drainage. With this observation, he used endoscopes to develop a surgical approach to relieve the obstruction in such a way that normal sinus physiology was preserved. Specifically, he demonstrated that even limited surgical procedures directed toward the osteomeatal complex and the anterior ethmoid air cells could relieve obstruction of drainage from the frontal and maxillary sinuses. This philosophy was markedly different from the ablative sinus procedures advocated in the past, such as Caldwell-Luc, in that cilia and sinus mucosal function were preserved. Hence these procedures were termed functional endoscopic sinus surgery (FESS); Stammberger and Kennedy further refined these techniques in the 1980s.
Anatomy and Physiology of the Sinonasal Tract
The frontal, maxillary, ethmoid, and sphenoid sinuses are formed early in development as evaginations of nasal respiratory mucosa into the facial bones. The ethmoid sinus develops into a labyrinth of 3 to 15 small air cells. In contrast, the other sinuses exist as a single bony cavity on each side of the facial skeleton. The ethmoid and maxillary sinuses are present at birth and can be imaged in infancy. The frontal sinuses develop anatomically by 12 months and can be evaluated radiographically at 4 to 6 years of age. Sphenoid sinuses begin to develop in children by the age of 3 years but cannot be imaged until they are 9 years or 10 years of age. The point at which mucosal outpouching occurs persists as the sinus ostium, through which the sinus drains (3).
Diagnostic rhinoscopy offers a wealth of information regarding the distribution of inflammatory foci within the sinonasal labyrinth and the associated anatomic variations that may impair physiologic sinus drainage. It is usually performed in an office setting with the aid of topical decongestants and topical anesthesia. It is essentially an extension of the physical examination that helps confirm the diagnosis, gain insight into the pathophysiologic factors at work, and guide medical or surgical therapy. The principles of diagnostic and therapeutic rhinoscopy are based on a firm understanding of
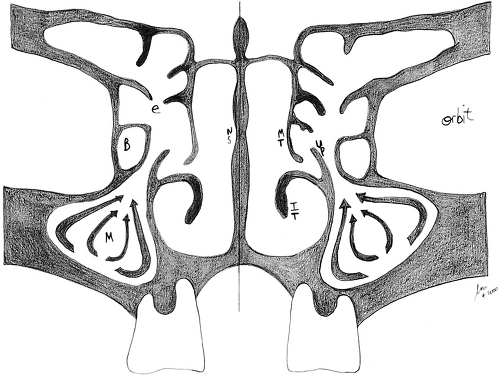
the anatomy and physiology of the nose and sinuses (Fig. 12.1). The lateral nasal walls are each flanked by three turbinate bones, designated the superior, middle, and inferior turbinates. The region under each turbinate is known respectively as the superior, middle, and inferior meatus. The anatomy of the lateral nasal wall is of key importance for the understanding of sinonasal physiology and the principles of FESS, because the ostium of each sinus drains into an anatomically specific location. The frontal, maxillary, and anterior ethmoid sinuses drain on the lateral nasal wall in a region within the middle meatus, known as the OMC. This is an anatomically narrow space where even minimal mucosal disease can result in impairment of drainage from any of these sinuses.
the anatomy and physiology of the nose and sinuses (Fig. 12.1). The lateral nasal walls are each flanked by three turbinate bones, designated the superior, middle, and inferior turbinates. The region under each turbinate is known respectively as the superior, middle, and inferior meatus. The anatomy of the lateral nasal wall is of key importance for the understanding of sinonasal physiology and the principles of FESS, because the ostium of each sinus drains into an anatomically specific location. The frontal, maxillary, and anterior ethmoid sinuses drain on the lateral nasal wall in a region within the middle meatus, known as the OMC. This is an anatomically narrow space where even minimal mucosal disease can result in impairment of drainage from any of these sinuses.
The posterior ethmoid sinuses drain into the superior meatus but are often aerated via the middle meatus during FESS. The sphenoid sinus drains into a region known as the sphenoethmoidal recess, which lies at the junction of the sphenoid and ethmoid bones in the posterior superior nasal cavity. The nasolacrimal duct courses anteriorly to the maxillary sinus ostium and drains into the inferior meatus. The ethmoid bone is the most important component of the OMC and lateral nasal wall. It is a T-shaped structure, of which the horizontal portion forms the cribriform plate of the skull base. The vertical part forms most of the lateral nasal wall and consists of the superior and middle turbinates, as well as the ethmoid sinus labyrinth. Within the middle meatus, a sickle-shaped projection of the ethmoid bone, known as the uncinate process, forms a recess, called the infundibulum, into which the maxillary sinus drains (4).
A collection of anterior ethmoid air cells forms a bulla, which is suspended from the remainder of the ethmoid bone, and hangs just superiorly to the opening of the infundibulum into the meatus. The drainage duct for the frontal sinus courses inferiorly such that its ostium lies posterior and often just medial to the anterior-most ethmoid air cell. Therefore, the main components of the OMC are the maxillary sinus ostium/infundibulum, the anterior ethmoid cells/bulla, and the frontal recess. The infundibulum and frontal recess exist as narrow clefts; thus, it is possible that minimal inflammation of the adjacent ethmoidal mucosa can result in secondary obstruction of the maxillary and frontal sinuses.
The paranasal sinuses are lined by pseudostratified-ciliated columnar epithelium, over which lays a thin blanket of mucus. The cilia beat in a predetermined direction such that the mucous layer is directed toward the natural ostium and into the appropriate meatus of the nasal airway. This is the process by which microbial organisms and debris are cleared from the sinuses (4). This principle of mucociliary flow is analogous to the “mucociliary elevator” described for the tracheobronchial tree. The maxillary ostium and infundibulum are located superior and medial to the maxillary sinus cavity itself. Therefore, mucociliary clearance in the maxillary sinus must overcome the tendency for mucus to pool in dependent areas of the sinus. Successful FESS entails enhancement of drainage via the natural ostium.
Antrostomies placed in dependent portions of the sinus are less effective because they interfere with normal sinus physiology.
Antrostomies placed in dependent portions of the sinus are less effective because they interfere with normal sinus physiology.
Pathophysiology of Chronic Rhinosinusitis
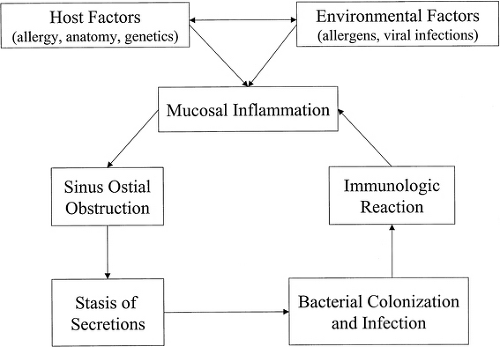
The American Academy of Otolaryngology–Head and Neck Surgery Task Force on Rhinosinusitis (5) defines sinusitis as “inflammation of the mucosa of the nose and paranasal sinuses.” Rhinosinusitis, rather than sinusitis, is the more appropriate term, because sinus inflammation is often preceded by rhinitis and rarely occurs without coexisting rhinitis. Primary inflammation of the nasal membranes, specifically in the region of the OMC, results in impaired sinus drainage and bacterial superinfection, resulting in further inflammation (Fig. 12.2). In most patients, a variety of host and environmental factors serve to precipitate initial inflammatory changes. Host factors include systemic processes such as allergic and immunologic conditions, various genetic disorders (e.g., immotile cilia syndrome and cystic fibrosis), and metabolic/endocrine disorders. Host variations in sinonasal anatomy also occur, predisposing some to ostial obstruction with even minimal degrees of mucosal inflammation. Neoplasms of the nose and maxilla and nasal polyps also may cause anatomic obstruction. Environmental factors play a vital role, including infectious agents, allergens, medications, trauma, and noxious fumes such as tobacco smoke (5). The pathophysiology of CRS can be influenced by sinonasal anatomy, infection, and allergic/immunologic disorders. Rhinoscopy can provide significant insight into the relative importance of these elements in each individual patient. The infectious, allergic, and immunologic elements of CRS are typically subjected to intense pharmacologic treatment. It should be noted that the specific immunologic factors that predispose a patient to CRS is an area of active investigation, and current evidence implicates many potential factors beyond IgE-mediated allergy. The disease process is better understood as a clinical syndrome caused by inflammatory etiologies, rather than as an infectious disease. Microorganisms, however, play a significant role in the progression and exacerbation of the condition.
Some of these underlying inflammatory factors may predispose the CRS patient to polyp growth, and prevailing thought suggests that polyp growth is associated with a CD4+ T helper 2 (TH2) cytokine profile (interleukins such as IL-4, IL-5, IL-13) and eosinophilic inflammation, while nonpolypoid CRS tends to exhibit a CD4+ TH1 cytokine profile and neutrophilic inflammation. This distinction may have significant therapeutic implications (6).
Anatomic Influences
Anatomic variations can contribute to the symptomatology in patients with CRS; these variations would include congenital, surgical, traumatic, or postinflammatory alterations in the normal structure. Common variations include septal deviations and spurs, and hypertrophic, pneumatized (concha bullosa), bent, or flattened turbinates. These entities create inherent anatomic narrowing of the bony channels through which mucus and air flow. When superimposed on inflammatory edema of the overlying mucosa, these factors may initiate the cascade of events resulting in symptoms of nasal airway obstruction, and possibly limitation of mucocilliary flow. Anatomic obstruction may also be precipitated or exacerbated by mass effect from inflammatory polyps, and occasionally, true neoplasms are encountered.
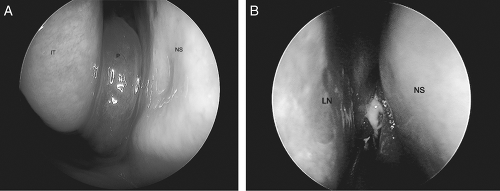
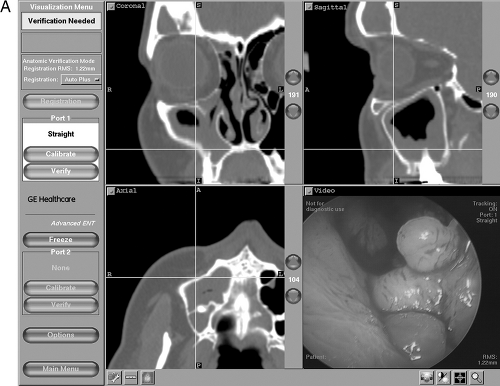
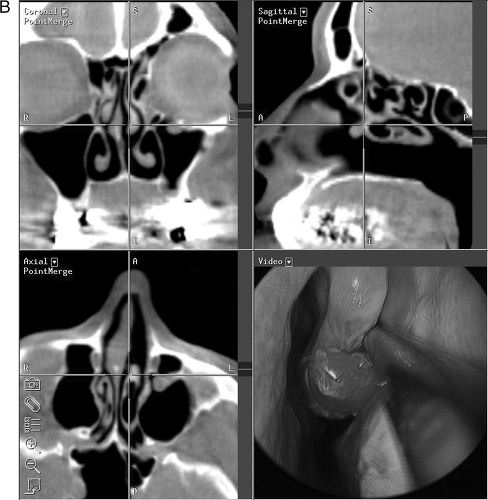
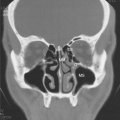
Accessory sinus ostia may result in recirculation of mucus with diminished net mucociliary clearance. These factors may, theoretically, induce progression to CRS, although the exact relationship between various anatomic factors and the development of CRS has been difficult to demonstrate statistically. Diagnostic nasal endoscopy is an important modality to elucidate which of these entities (or combination thereof) may be implicated in any individual patient with CRS. A sample of commonly encountered endoscopic findings is illustrated in Fig. 12.3. Although paranasal sinus imaging is beyond the scope of this chapter, it should be noted that computed tomography (CT) scan of the sinuses and nasal endoscopy are complimentary diagnostic modalities, as illustrated in Fig. 12.4.
Infection
Rhinosinusitis often is preceded by an acute viral illness such as the common cold (5). This leads to mucosal swelling, obstruction of sinus outflow, stasis of secretions, and subsequent bacterial colonization and infection. From the acute phase, four possible courses are possible. These include resolution, progression with
adverse sequelae such as orbital or intracranial infection, development of silent CRS, or the development of symptomatic CRS. In turn, CRS may undergo resolution, persistence, or the development of adverse sequelae, depending on the host and environmental variables at work (5). In the chronic persistent state, microbial colonization and infection lead to additional inflammation, further exacerbating the process.
adverse sequelae such as orbital or intracranial infection, development of silent CRS, or the development of symptomatic CRS. In turn, CRS may undergo resolution, persistence, or the development of adverse sequelae, depending on the host and environmental variables at work (5). In the chronic persistent state, microbial colonization and infection lead to additional inflammation, further exacerbating the process.
With the development of symptomatic CRS, multiple bacteria usually are cultured, including anaerobes and β-lactamase–producing organisms (7,8). Some are apparently pathogens, whereas others are opportunistic, nonvirulent strains. Cultures obtained under rhinoscopic guidance or those obtained from tissue removed at surgery may help to guide appropriate antibiotic selection. Histopathologic studies of sinus mucosa taken from patients with CRS do not generally demonstrate bacterial tissue invasion. A pronounced inflammatory response with a dense lymphocytic infiltrate typically is seen, at least in part, as a response to the bacteria. The symptomatology associated with CRS is probably a result of this inflammatory reaction.
Allergic Rhinitis
The exact incidence of allergy in patients with CRS is unclear. It is reported that 38% to 67% of patients with CRS who require FESS have comorbid allergic rhinitis (9). This observation is true in children as well as adults. In susceptible individuals, provocation by airborne inhalant allergens triggers the release of mediators from mast cells that reside in the nasal mucosa. Immunoglobulin E (IgE)-mediated inflammation may hypothetically lead to mucosal edema and osteomeatal obstruction, with secondary sinusitis. The early phase is primarily mediated by histamine and leukotrienes, whereas late-phase reactions result from cytokines and cellular responses. It should be noted that the prevalence of positive immediate skin testing is sometimes less than what would be expected intuitively, so that the overall impact of systemic IgE-mediated disease is still unclear. Nonallergic rhinitis, including vasomotor rhinitis, also can result in osteomeatal obstruction and secondary sinusitis.
Nasal Polyps
The exact etiology of nasal polyps (NPs) is unknown, and likely mutifactorial. NP growth is associated with high-grade chronic sinonasal inflammation in susceptible individuals. Degranulated eosinophils often are present (10); such eosinophils are known to secrete IL-5, IL-3, and granulocyte-macrophage colony-stimulating factor (GMC-SF), all of which are eosinophil growth factors. NPs also can be associated with specific disorders, such as aspirin-sensitive asthma and cystic fibrosis (CF).
The latter diagnosis should be excluded by chloride sweat test in any pediatric patient with NPs (2). The relationship of NPs to allergic rhinitis is uncertain (11). Because NPs observed on rhinoscopic examination may coexist with specific underlying disorders such as asthma, CF, or allergic fungal sinusitis (AFS), their detection may indicate the need for further evaluation of these conditions (12).
The latter diagnosis should be excluded by chloride sweat test in any pediatric patient with NPs (2). The relationship of NPs to allergic rhinitis is uncertain (11). Because NPs observed on rhinoscopic examination may coexist with specific underlying disorders such as asthma, CF, or allergic fungal sinusitis (AFS), their detection may indicate the need for further evaluation of these conditions (12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree