Chronic granulomatous disease (CGD) is a paradigm for nonlymphoid primary immune defects, and has guided elucidation of oxygen metabolism in the phagocyte, vasculature, and brain. It has been in the forefront of the development of antimicrobial prophylaxis before the advent of advanced HIV and before its routine use in neutropenia. It has been an attractive target for gene therapy and bone marrow transplantation for nonmalignant diseases. Therefore, CGD is worthy of attention for its historical interest and because it is a disease for which expert management is imperative.
Key points
- •
Chronic granulomatous disease (CGD) is a single gene defect that can be reconstituted in vitro and does not require complete correction to be effective, as proven by the normal lives of many X-linked carriers, and by the stable chimeras generated in some transplant protocols.
- •
Unlike the case with severe combined immunodeficiency, corrected CGD cells do not have a growth or survival advantage in the marrow or tissue. Therefore, selection and augmentation of those cells is difficult.
- •
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is active outside the neutrophil, such as in nuclear factor κβ signaling, liver damage from carcinogens, and the arterial vasculature.
- •
NADPH oxidase somatic and hematopoietic activity is involved in strokes and pulmonary vascular permeability. NADPH contributes to long-term potentiation of memory and may be related to IQ.
- •
NADPH oxidase is clearly active in many more sites than just phagocytes, suggesting that CGD is more complex and can teach about more than just infections and bone marrow transplants alone.
Chronic granulomatous disease (CGD) was first described in 1954 and 1957 as recurrent infections occurring in the setting of hypergammaglobulinemia, as opposed to the disease then recently recognized by Bruton, in which infections were associated with hypogammaglobulinemia. The disease was not well characterized until 1959, when it was initially termed fatal granulomatous disease of childhood , but it is now simply referred to as chronic granulomatous disease . Originally thought to be only an X-linked disease, its recognition in girls in 1968 also led to the determination of autosomal recessive forms. Over almost 60 years, CGD has evolved from a disease of early fatality to one of effective management with high survival. CGD is a paradigm for nonlymphoid primary immune defects, and has guided elucidation of oxygen metabolism in the phagocyte, vasculature, and brain. It has been in the forefront of the development of antimicrobial prophylaxis before the advent of advanced HIV and before its routine use in neutropenia. It has been an attractive target for gene therapy and bone marrow transplantation for nonmalignant diseases. Therefore, CGD is worthy of attention for its historical interest and because it is a disease for which expert management is imperative.
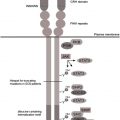
Multiple separate proteins contribute to the intact nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, mutations in 5 of which lead to the single syndrome of CGD. NADPH oxidase catalyzes the transfer of an electron from cytoplasmic NADPH to molecular oxygen (6; OMIM# 306400 , 233690 , 233700 , 233710 , 601488 ), thereby oxidizing NADPH and leading to the name NADPH oxidase . Although impairments of the NADPH oxidase typically present as phagocyte defects, in fact only gp91 phox is relatively phagocyte-specific, whereas the other autosomal components are also expressed elsewhere. The components are broken into membrane-bound (cytochrome b558, composed of gp91 phox and p22 phox ) and cytosolic (p47 phox , p67 phox , and p40 phox ) structures. The subunits gp91 phox and p22 phox require each other for expression in the phagocyte; however, because p22 phox is expressed in other tissues and gp91 phox is not, p22 phox and the other members of the NADPH oxidase join with other partners in the other tissues, which are other members of the Nox family of proteins. Therefore, individuals who have autosomal recessive forms of CGD may also have other subtle abnormalities, such as vascular disease and diabetes in p47 phox -deficient CGD or perhaps inflammatory bowel disease in p40 phox -deficient CGD.
Activation of the NADPH oxidase is a carefully choreographed process. On cellular activation, such as ingestion of bacteria of fungi, the cytosolic components p47 phox and p67 phox are phosphorylated and bind tightly together. The secondary (specific) granules, which contain the cytochrome complex (gp91 phox and p22 phox ) fuse with the phagolysosome, followed by the primary (azurophilic) granules, which contain the antibacterial peptides neutrophil elastase and cathepsin G. This process embeds the cytochrome in the wall of the phagolysosome and the antibacterial peptides inside it. The cytoplasmic complex of p47 phox and p67 phox in association with p40 phox and RAC2 combine with the cytochrome to form the intact NADPH oxidase, which is oriented into the internal aspect of the phagolysosome (this process can also occur on the plasma membrane focused outside the cell). An electron is then taken from cytoplasmic NADPH and donated to molecular oxygen inside the phagolysosome, leading to the formation of superoxide. In the presence of superoxide dismutase, this is converted to hydrogen peroxide, which, in the presence of myeloperoxidase and chlorine in the phagolysosome, is converted to bleach. Although the metabolites of superoxide themselves can contribute to bacterial killing, the generation of superoxide has broader implications. With the generation of superoxide, a charge is imparted to the phagolysosome that is rectified by the rapid influx of potassium ions. This potassium influx leads to activation of the now-intraphagosomal peptides, which mediate microbial killing. Therefore, reactive oxidants are working more as intracellular signaling molecules, leading to activation of other nonoxidative pathways in addition to causing killing directly. Thus, a spectrum of microbicidal activity can be regulated by NADPH oxidase activity, rather than distinct oxidative and nonoxidative pathways and mechanisms.
In addition to activation of intracellular antimicrobial peptides, the NADPH oxidase is required to activate neutrophil extracellular traps (NETs), complex assemblies of DNA and antimicrobial peptides released from apoptotic neutrophils. Repair of the NADPH oxidase system with gene therapy in a patient with X-linked CGD led to reconstitution of NET function, proving that NET formation is impaired in CGD and dependent on NADPH function.
Mutations in all of the 5 structural genes of the NADPH oxidase have been found to cause CGD. Mutations in gp91 phox account for approximately 65% of cases, mutations in p47 phox account for approximately 25%, and the remainder are divided between p67 phox and p22 phox ; one case of p40 phox deficiency has been reported. No autosomal dominant cases of CGD have been reported. A large voluntary retrospective study in the United States and Europe suggested rates of CGD of around 1 in 200,000 to 1 in 250,000 live births. Rates in other countries vary depending on the ethnic practices and degrees of intermarriage: Sweden 1 in 450,000; Japan 1 in 300,000; Israeli Jews 1 in 218,000; Israeli Arabs 1 in 111,000. However, the relative rates of X-linked compared with recessive CGD are distinct. In many countries with high rates of consanguineous marriage, recessive CGD rates exceed X-linked rates. Clinically, X-linked gp91 phox -deficient CGD is more severe with earlier presentation and diagnosis, and more severe infections and earlier death than the p47 phox -deficient form.
Mechanistically, the level of residual superoxide production determines survival, at least in X-linked CGD. The rates of long-term survival are higher in those patients with X-linked CGD with higher residual superoxide production and lower in those with lower production. Molecularly, this is caused by the ability of the mutant protein to support superoxide production. Thus, mutations that lead to no protein production (nonsense mutations, deletions, certain splice defects) support no residual superoxide production and have the lowest level of survival. In contrast, those mutations that permit protein production and superoxide generation (missense mutations before amino acid 310 except histidine 222) are associated with higher survival rates. However, mutations at or beyond amino acid 310 are unable to support superoxide production, presumably because of the strict structural requirements of the intracellular domain of gp91 phox for the binding of NADPH and flavin adenine dinucleotide (FAD).
Infections of the lung, skin, lymph nodes, and liver are the most frequent first manifestations of CGD. In North America, most infections in CGD are from Staphylococcus aureus, Burkholderia cepacia complex, Serratia marcescens, Nocardia spp, and Aspergillus spp. In other parts of the world Salmonella , Bacille Calmette–Guérin (BCG), and tuberculosis are also important. Patients with CGD tend to develop severe localized BCG rather than disseminated BCGosis. Trimethoprim/sulfamethoxazole prophylaxis has reduced the frequency of bacterial infections in general and staphylococcal infections in particular. On prophylaxis, staphylococcal infections are essentially confined to the liver and cervical lymph nodes. Staphylococcal liver abscesses encountered in CGD are dense, caseous, and difficult to drain, and previously required surgery in almost all cases. However, recent studies have shown that the combination of corticosteroids and antibiotics alone are highly effective in CGD liver abscesses, allowing cure of liver abscesses without surgery. Until recently fungal infections, typically because of Aspergillus spp, were the leading cause of mortality in CGD. The advent of highly active antifungal therapy with the orally active azole antifungals itraconazole, voriconazole, and posaconazole has changed the face of fungal infections in CGD. Mortality from A fumigatus infection in CGD is now uncommon, and therefore mortality overall is diminished. However, the non- fumigatus Aspergillus spp, and some species of fungi other than Aspergillus remain difficult to treat and important contributors to mortality.
Overall survival in CGD is now thought to be approximately 90%, stretching well into adulthood. Patients diagnosed before the advent of antifungal azole agents had different outcomes, reflected by the poor survival of patients into their 30s and 40s in some series. Since the introduction of itraconazole in the late 1990s, its proof in antifungal prophylaxis in 2003, and the introduction of more active antifungals and antibacterials, mortality in CGD has plunged. Access to expert care is clearly important, as shown by a Japanese study with a 90% survival rate for patients followed up at single center. Similarly, Mouy and colleagues found an 8-year survival rate of 70.5% for children born before 1978 but a 92.9% survival rate for those born later. Before the introduction of oral antifungals Winkelstein and colleagues reported X-linked CGD mortality of approximately 5% per year, compared with 2% per year for the autosomal recessive varieties. van den Berg and colleagues found a 23% mortality in X-linked CGD and a 15% rate of mortality in autosomal recessive CGD over almost 50 years of European survey. Therefore, overall mortality from infection in CGD has been significant but will continue to improve with better therapies.
The morbidity of recurrent infections and inflammation, with their associated end-organ damage and their impact on the child and family, is a major issue. Several large studies found similar rates of infection of around 0.3 per year. That is, most patients are still experiencing at least one severe infection every 3 to 4 years, whether bacterial or fungal. The persistence of this rate may reflect a minimum inescapable environmental exposure, or the complexity of maintaining long-term prophylaxis over a lifetime with a disease that is only intermittently reinforced.
CGD is remarkable because of its very narrow but profound spectrum of infection susceptibility. B cepacia complex organisms are common causes of pneumonia and, infrequently, sepsis. The closely related B gladioli has also been described in CGD. Chromobacterium violaceum is found in brackish waters, such as those around the Gulf of Mexico in the United States, and causes sepsis in CGD. Francisella philomiragia causes sepsis in CGD and is also found in brackish waters, such as the Chesapeake Bay, Long Island Sound, and around Nova Scotia. Granulibacter bethesdensis is a novel gram-negative rod that causes chronic necrotizing lymphadenitis and can cause sepsis in CGD. It can have latent and active phases, similar to tuberculosis, and has been identified in the United States, Panama, and Spain, suggesting wide distribution. Although the rate of seropositivity for this organism is approximately 50% in patients with CGD, most of whom have not had recognized infections, this rate is around 25% in patients without CGD, suggesting broad exposure and the possibility of a clinical syndrome yet to be identified.
Fungal infections are critically important to recognize in CGD. Several are characteristic of CGD and virtually never encountered in other diseases: A nidulans, Paecilomyces variotii and P lilacinus , and Neosartorya udagawae . These organisms are highly pathogenic in those infected with CGD but not in any other patient group, including transplant recipients. In contrast to these filamentous molds that are virtually pathognomonic for CGD, the endemic dimorphic mold infections histoplasmosis, blastomycosis, and coccidioidomycosis do not occur in CGD, nor does cryptococcosis. Mucormycosis occurs in CGD but only in the setting of significant immunosuppression. The molecular identification of infection should be vigorously pursued in patients with CGD, especially for fungal infections, because the identification of a non- fumigatus Aspergillus infection should prompt early consideration of therapeutic surgery.
Fungal elements elicit an exuberant inflammatory response in CGD regardless of whether they are live or dead. Mulch pneumonitis refers to acute inhalational exposure to aerosolized decayed organic matter, such as mulch, hay, or dead leaves. The clinical presentation is stereotypic and dramatic: a previously well child or adult spreads mulch, turns compost, or clears moldy leaves, inhaling numerous fungal spores and hyphae; 1 to 10 days later a syndrome similar to hypersensitivity pneumonitis begins with fever and dyspnea; chest radiographs show diffuse interstitial infiltrates; bronchoscopy is usually uninformative but may yield Aspergillus ; lung biopsy shows acute inflammation with necrotizing granulomata and fungi. The most successful treatments of this syndrome have been with simultaneous antifungals for the infection and steroids for the inflammation. The authors typically institute meropenem, voriconazole, and prednisone for this syndrome, because steroids seem to be crucial for reducing inflammation and allowing independent ventilation. This syndrome should be considered in all cases of Aspergillus pneumonitis, especially with acute onset and hypoxia, and therefore implies CGD as the underlying diagnosis. This syndrome occurs in patients with known CGD but also may be the presentation for disease, even in adults.
Inflammation in CGD is most prominent in the gastrointestinal and genitourinary tracts. Esophageal, jejunal, ileal, cecal, rectal, and perirectal involvement with granulomata mimicking Crohn disease have been described. Functional gastric outlet obstruction may be the initial presentation of CGD. In a large survey of patients with CGD followed at the National Institutes of Health, 43% of those with X-linked CGD had symptomatic, biopsy-proven inflammatory bowel disease (IBD), compared with only 11% of p47 phox -deficient patients. However, growth rates were equally diminished to less than the mean in IBD-affected and unaffected patients. Whether the mild growth retardation seen in most patients with CGD was from IBD in all cases, or from some other CGD-associated feature of the disease is unknown, because biopsies were only performed in symptomatic patients. Growth and growth rates in CGD recovered after bone marrow transplantation, regardless of antecedent IBD. Although IBD may involve any part of the gastrointestinal tract in patients with CGD, perirectal disease is especially common.
Treatment of CGD IBD is often long-term and difficult, and the disease is prone to relapse. Steroids are effective but may cause growth retardation, osteoporosis, and infection risk. However, at the doses typically used in CGD for maintenance, infections are rarely an issue. In contrast, although tumor necrosis factor (TNF)-α–blocking agents are highly effective and rapidly suppress bowel symptoms, they confer a high risk of infection and death and should be carefully avoided in patients with CGD. TNF-α inhibitors predispose to characteristic CGD pathogens, only more severe episodes. The authors’ current practice is to initiate therapy for proven IBD in CGD with prednisone at 1 mg/kg/d for 1 to 2 weeks and then slowly taper to 0.1 to 0.25 mg/kg/d over 1 to 2 months. Sometimes prednisone can be stopped in children, but the relapse rate is very high, and re-treatment typically requires reinitiation of the higher dose. Therefore, after the first recurrence or relapse the authors usually add an antimetabolite, such as azathioprine (Imuran), along with salicylic acid derivatives. Local treatments such as steroid enemas and rectal creams are also highly effective.
Liver involvement in CGD is pronounced and important. Liver abscesses occur in approximately 35% of patients and until recently have been difficult to treat without surgery. With surgery, cure of liver abscess is common, but unfortunately so is reinfection, typically with a different organism from the previous one. Whether certain patients with CGD are simply predisposed to liver abscesses or having had a previous liver abscess alters hepatic architecture and blood flow in a way that makes subsequent infection more likely remains unclear. High rates of portal venopathy and nodular regenerative hyperplasia may contribute to portal hypertension, splenomegaly, and splenic sequestration. This latter point is noteworthy, because the decline in platelet count is linked to splenomegaly and is also a strong predictor of mortality in CGD. Chronic drug effects, liver enzyme elevations, and recurrent infections are obvious risks for liver dysfunction. In this regard, whether the predilection for recurrent liver abscesses is partly caused by surgery, with its hepatic scarring and altered blood flow, is unclear. However, avoiding surgery when possible seems prudent, and the recent demonstration of the effectiveness of steroids and antibiotics alone in the resolution of liver abscess offers an alternative treatment.
Genitourinary manifestations of CGD are also common and include bladder granulomata, ureteral obstruction, and urinary tract infection, typically in those with gp91 phox and p22 phox deficiencies. Eosinophilic cystitis has also been described in CGD. Genitourinary complications are also highly steroid responsive. Inflammatory masses in CGD can mimic tumors and should be considered.
The diagnosis of CGD is usually made by direct measurement of superoxide production, ferricytochrome c reduction, chemiluminescence, nitroblue tetrazolium (NBT) reduction, or dihydrorhodamine oxidation (DHR). DHR is preferable because of its relative ease of use, its ability to distinguish X-linked from autosomal patterns of CGD on flow cytometry, its sensitivity to even very low numbers of functional neutrophils, and its utility in predicting the residual superoxide activity of the patient’s neutrophils. The 2 conditions known to give a falsely abnormal DHR are myeloperoxidase deficiency and SAPHO syndrome. In myeloperoxidase deficiency, the DHR tracing can look like that of X-linked CGD, whereas the NBT and ferricytochrome c results are normal. This finding is attributed to intracellular (DHR) compared with extracellular (NBT) superoxide release and dye activation. Glucose 6-phosphate dehydrogenase (G6PD) deficiency may also lead to a decreased respiratory burst and increased susceptibility to bacterial infections. However, G6PD deficiency is most often associated with some degree of hemolytic anemia, whereas CGD is not.
Female carriers of X-linked CGD have 2 populations of phagocytes: 1 that produces superoxide and 1 that does not, yielding a characteristic mosaic pattern on oxidative testing. Infections are infrequent unless the normal neutrophils are less than 10%, and even then are uncommon. However, cases of severe skewing of X-chromosome inactivation have been reported, in which women have virtually no detectable normal cells; these carriers are at risk for CGD-type infections. Reports suggest that the balance of wild-type to mutant cells may vary over time in the same woman, but this has not been rigorously proven, as likely as it may seem. Discoid lupus erythematosus–like lesions, aphthous ulcers, and photosensitive rashes have been seen in gp91 phox carriers. Similarly, screening of patients with discoid lupus erythematosus detected a significant number of previously unsuspected CGD carriers.
Immunoblot and flow cytometry can be used to infer the specific genotype, but molecular determination of specific mutations is necessary for prenatal diagnosis. A robust genotype/phenotype correlation has been shown for mutations that permit residual superoxide production compared with those that do not. Male sex, earlier age at presentation, and increased severity of disease suggest X-linked disease, but these are only rough guides. The precise gene defect should probably be determined in all cases, because it is a strong predictor of survival. Autosomal recessive p47 phox -deficient CGD has a significantly better prognosis than X-linked disease.
Effective management of CGD is predicated on prophylactic antibiotics and antifungals and interferon (IFN)-γ, along with management of acute infections as they occur. Prophylactic trimethoprim/sulfamethoxazole (5 mg/kg/d trimethoprim divided twice daily) reduces the frequency of major infections from approximately once every year to once every 3.5 years, reducing staphylococcal and skin infections without increasing in the frequency of serious fungal infections in CGD. Itraconazole prophylaxis prevents fungal infections in CGD (100 mg daily for patients aged <13 y or weighing <50 kg; 200 mg daily for those aged >13 y or weighing >50 kg). IFN-γ was shown in a large, multinational, multicenter, placebo-controlled study to reduce the number and severity of infections in CGD by 70% compared with placebo regardless of inheritance pattern, sex, or use of prophylactic antibiotics. Systemic IFN-γ also augmented neutrophil activity against Aspergillus conidia in vitro. Furthermore, in a study of IFN-γ in CGD mice, infections were reduced. However, a retrospective Italian study detected no benefit to the addition of IFN-γ beyond that attributed to antibacterial and antifungals alone. Long-term follow-up of the large prospective trials suggests sustained benefit. The authors use trimethoprim/sulfamethoxazole, itraconazole, and IFN-γ (50 μg/m 2 ) in the treatment of CGD.
Bone marrow transplantation can lead to stable remission of CGD. Regimens ranging from full myeloablation to nonmyeloablative conditioning lead to cure of CGD. Even in the setting of refractory fungal infection, bone marrow transplantation has been effective. Nonmyeloablative transplants in CGD have been more successful in children than in adults. Although bone marrow transplantation is an attractive option for the definitive cure of CGD, survival without bone marrow transplantation is roughly comparable, but attended by continuing CGD morbidities, such as bowel disease and mildly reduced growth.
CGD is a group of single gene defects that can be reconstituted in vitro and does not require complete correction to be effective, as proven by the normal lives of many X-linked carriers, and by the stable chimeras generated in some transplant protocols. Unlike the case with severe combined immunodeficiency, corrected CGD cells do not have a growth or survival advantage in the marrow or tissue. Therefore, in vivo selection and augmentation of those cells is difficult.
NADPH oxidase is active outside the neutrophil, such as in nuclear factor κβ signaling, liver damage from carcinogens, and the arterial vasculature. NADPH oxidase somatic and hematopoietic activity is involved in strokes and pulmonary vascular permeability. NADPH contributes to long-term potentiation of memory and may be related to IQ. Therefore, NADPH oxidase is clearly active in many more sites than just phagocytes, suggesting that CGD is more complex and can teach about more than just infections and bone marrow transplants alone.
This work supported by the Division of Intramural Research , National Institute of Allergy and Infectious Diseases , National Institutes of Health .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree