Chronic obstructive pulmonary disease and acid base
Chronic obstructive pulmonary disorder (COPD) is a narrowing of the airways, leading to poor lung function and severe airflow obstruction (low FEV1). The cause of COPD is not clear, although there are several contributing factors such as smoking, exposure to workplace dust and particulates, air pollution and genetic factors (in about 2% of cases). It can also partly result from an autoimmune condition following prolonged inflammation, as seen in other autoimmune conditions.
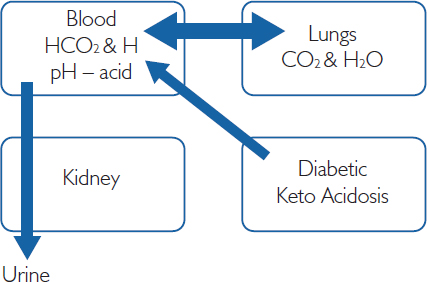
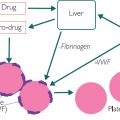
Figure 12.1: COPD Interactions
Patients with COPD may be more susceptible to upper and lower pulmonary infections – hence the importance of vaccinations. White blood cell count (WBC) may be a helpful marker for infection, as would the presence of a functional antibody (Ab) following vaccination.
Given the restriction in oxygen caused by COPD, the patient may develop polycythaemia with a raised red blood cell count (RBC), especially if they also smoke. However, in practice, in an elderly patient, the hypoxia-induced production of erythropoietin (EPO) may be mitigated by poor renal function (with an estimated glomerular filtration rate <60) and raised RBC may not occur, as EPO is made by the kidney.
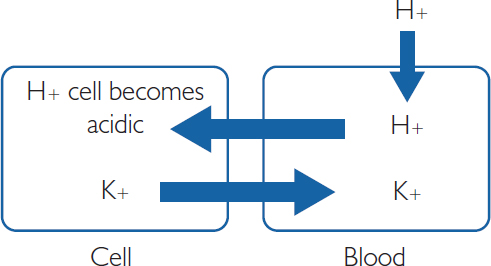
Figure 12.2: Consequences of Acidosis
In addition, tuberculosis may need to be excluded in COPD patients. It may also be appropriate to screen for alpha-1-antitrypsin, which provides important protection from attack by white cells. White cells, particularly neutrophils, use potent enzymes to destroy bacteria. To protect our own cells from attack, we make alpha-1-antitrypsin, which can block or inhibit these enzymes. In some autoimmune conditions and COPD, the patient may have a deficiency or lack of alpha-1-antitrypsin and the raised neutrophils can therefore cause more damage.
Acid base measures
Acid base measures are helpful in COPD patients, as well as in those with diabetes or drug overdose and those taking proton pump inhibitors. Acid and alkaline conditions are determined by the concentration of H ions (protons). A high H+ concentration is a low acid pH, and a low H+ concentration is a high alkaline pH. Most enzymes work within a narrow range of pH (around 7.4) to control these two systems, metabolic and respiratory, using the kidney and lungs respectively to ensure that this pH is maintained. Changes in H+ concentrations are controlled by buffers.
The equation
H+ + HCO3– (metabolic)  CO2 + H2O (respiratory)
CO2 + H2O (respiratory)
is used to describe the bicarbonate (HCO3) buffer system. Thus, if the pH rises (more H+), this moves the equation to the right, producing more CO2, which is cleared by a subsequent increase in ventilation. Equally, a poor ventilation rate will increase CO2 and move the equation to the left and increase pH. This is why COPD patients often have respiratory acidosis.
These compensatory mechanisms also function within a particular timespan. Physiological buffers, such as the bicarbonate–carbonic acid buffering system, work immediately. Pulmonary compensation occurs next, within a few minutes. Finally, about six hours after sustained acidosis or alkalosis, renal compensation occurs. In certain patients, bone can also be used as a last resort, as it contains high levels of bicarbonates, although this disrupts bone density. Therefore patients with COPD, renal dysfunction (chronic kidney disease) and bone dysfunction (osteoporosis) may have a very limited buffering capacity and will need to be carefully assessed.
Acid base derangements
The most common acid base derangements are metabolic acidosis, metabolic alkalosis, respiratory acidosis and respiratory alkalosis.
In metabolic acidosis, the pH is low, meaning high H+ load. Causes of metabolic acidosis include lactic acidosis, diabetic ketoacidosis (ketone bodies are produced from fat, which are acidic), and loss of bicarbonate through severe diarrhoea or bicarbonate wasting through the kidneys or gastrointestinal (GI) tract. This is metabolic, partly because cells are producing excess acid and partly because the kidney attempts to clear H+. H+ in excess can move into the cells and cause K+ to be shunted out, leading to hyperkalaemia and a possible coronary heart disease event.
As HCO3– increases (usually as the result of excessive loss of metabolic acids), metabolic alkalosis occurs. Causes of metabolic alkalosis include Cushing’s syndrome, some diuretics, secretory adenoma of the colon, and exogenous steroids.
Respiratory acidosis (pH <7.35, PaCO2 >45 mm Hg) reflects alveolar hypoventilation. Given that the renal control of HCO3– is tightly controlled, large and prolonged changes of PaCO2 are required to increase pH. As discussed, this is usually seen in primary care as COPD, and in acute care as brainstem injury from acute ingestion of opioids. Supportive O2 treatments may be used, or in acute cases of overdose corrective therapies like intravenous naloxone may be considered.
Respiratory alkalosis usually occurs as a result of hyperventilation, often caused by mechanical over-ventilation, hepatic disease, ‘panic attacks’, pregnancy and septicaemia. Treatments are usually corrective, such as controlling breathing. However, the patient should be monitored to prevent subsequent metabolic acidosis.
Table 12.1: Sample acid base results
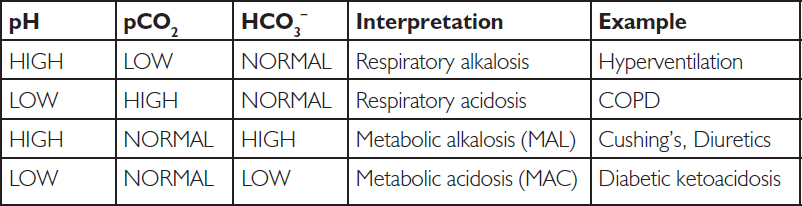
It is also possible to have a mixed acid base. For example, a patient who has lactic acidosis (metabolic) and COPD (respiratory) might have the following results: pH 7.12, CO2 55, HCO3– 14, and so on.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree