Major Concepts
Symptoms
Chagas’ disease is a severe, debilitating, and sometimes fatal disease found throughout impoverished areas of Latin America, and imported as well as endemic cases of this illness are increasingly turning up in the southern United States. The disease course involves an often asymptomatic acute phase, a 20- to 30-year latent phase, and a pathogenic chronic phase. The chronic phase is characterized by either damage to the heart or by enlargement of the esophagus and colon. Heart pathology includes cardiomyopathy with or without congestive heart failure, arrhythmia, right bundle blockage, and sudden cardiac death. Enlargement of the esophagus and colon leads to difficulty in swallowing, malnutrition, constipation, and abdominal pain.
Infection
Chagas’ disease is caused by the parasitic protozoan Trypanosoma cruzi and is transmitted to humans via the feces of infected triatomine bugs, blood transfusions, organ transplants, or transplacentally. T. cruzi and its close relatives, the causative agents of African sleeping sickness and leishmaniasis, have complex life cycles involving several life stages and insect as well as human hosts. The infective form of the parasite for humans is the blood-dwelling metacyclic trypomastigote, and the replicative form is the amastigote, which divides inside cells, such as macrophages. The infective form of the parasite for the insect host is the bloodstream trypomastigote, and the replicative form is the epimastigote.
Immune Response
Parasite-induced immunosuppression occurs during the acute phase of Chagas’ disease, with decreased production of cytokines and expression of cell surface molecules. Lymphocyte growth is also inhibited. Suppressive molecules produced by the parasite may induce production of regulatory cytokines. Numbers of CD8+ killer cells are increased during Chagas’ disease, possibly augmenting heart pathology. Autoimmune responses may also play a role in heart damage.
Protection
Few compounds are currently used to treat Chagas’ disease. These are most effective if administered during the acute phase of the disease and are not often curative. Prevention may be the best protective strategy and primarily involves decreasing human contact with infected insects by improving building construction materials or spraying insecticides in dwellings or structures housing domestic animals. Screening the blood supply for contamination could also decrease infection via transfusion. These strategies are often expensive, however, and may be impractical for the poverty-stricken areas most affected by this disease.
An estimated 20 million people in the Western Hemisphere are infected with Trypanosoma cruzi, the causative agent of Chagas’ disease, and many additional millions may be at risk. Some 50,000 to 200,000 new infections occur each year. During the chronic phase of illness, infected individuals suffer progressive debilitating damage to the heart or digestive system that is often lethal. As a result of reduced productivity, disability, and death, Chagas’ disease is responsible for the loss of 670,000 disability-adjusted life years annually. It is a neglected illness arising out of poverty and in turn leads to even greater poverty in the endemic regions in a vicious circle.
Originally believed to be restricted to tropical and subtropical regions of South and Central America and Mexico and to persons originating in or visiting those areas, increasing numbers of reports have now confirmed the presence of T. cruzi in invertebrate and vertebrate hosts, including wild and domestic animals, in the southern United States. As a result of immigration and travel of vacationing and military persons to high-risk areas in Latin America, about 500,000 persons in the United States are believed to be infected. These individuals may infect others through blood or organ donation or may transmit the disease to their offspring transplacentally. Endemic transmission of Chagas’ disease involving infected insects and mammalian reservoir species in the United States is becoming an increasing concern as well. Because American physicians are generally not familiar with Chagas’ disease, many cases of this serious illness remain undetected and are attributed to other causes of cardiac disease.
The Brazilian physician Carlos Chagas discovered the causative agent of the disease that now bears his name in its invertebrate host and then in domestic animals (dogs and cats) and humans. He dubbed it Schizotrypanum cruzi (later T. cruzi) in honor of his mentor, Oswaldo Cruz, and described the disease it caused, found hosts among wild animal populations, and traced the parasite’s life cycle. Chagas first noted the infection in a 2-year old girl, Berenice, in 1909. At age 53, Berenice remained infected but displayed no symptoms of chronic infection, such as cardiac or digestive manifestations. She died at age 73, apparently of heart disease.
The noted biologist Charles Darwin is thought to have acquired Chagas’ disease during his historic journey on the H.M.S. Beagle. He recorded in March 1835 having being bitten by the disease vector, which he had previously studied: “At night I experienced an attack (for it deserves no less name) of the Benchuca, a species of Reduvius, the great black bug of the Pampas. It is most disgusting to feel soft wingless insects about one inch long crawling over one’s body. Before sucking, they are quite thin but afterwards they become round and bloated with blood, and in this state they are easily crushed.”
From several years thereafter, Darwin may have been in the latent phase of disease, but for the following 25 years, he wrote of heart palpitations, lassitude, extreme fatigue, and occasional trembling, flatulence, and vomiting. Darwin’s health later improved until early 1882, when he experienced the first of four heart attacks in the last four months of his life. Whether Darwin’s mysterious illness was indeed Chagas’ disease remains a matter of speculation; however, it is similar to that seen in Berenice.
The Phases of Chagas’ Disease
Chagas’ disease (American trypanosomiasis), in its chronic form, is a highly pathogenic and often fatal illness for which no effective treatment is currently available. The disease may be divided into three phases—acute, latent, and chronic—which are not universal among infected individuals. The development of chronic-phase symptoms frequently signals that death will follow within one year. Disease incidence is greatest among individuals over the age of 35 years and is equally distributed among males and females. Frequency of infection is increasing in both the southern and northern parts of the Western Hemisphere.
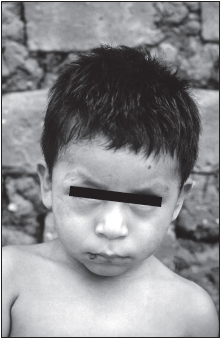
The acute phase occurs early after infection and is often inapparent, manifesting itself most frequently among children. Most infected persons do not seek medical attention during this stage. It presents itself as either an indurated skin lesion (chagoma) or a unilateral edema of the eyelid, conjunctivitis, and an enlarged satellite lymph node (Romaña’s sign). Parasitemia (the presence of demonstrable parasites in the blood) may be present during this time, diminishing within two to three months. Fever, hepatosplenomegaly, lymphadenopathy, lymphocytosis, electrocardiograph abnormalities, heart failure, and meningoencephalitis may occur at this time. Infants and very young children are most susceptible to development of meningoencephalitis, which may be fatal. The mortality rate in the acute stage is 5% to 10% among individuals aged 4 years or younger who are untreated (90% of those infected in some regions). In most individuals, however, the acute phase symptoms resolve spontaneously after four to eight weeks.
The length of the latent phase of the disease is variable and may last 20 to 30 years. For approximately 30% of infected persons, this is followed by the more severe chronic phase in which the majority of the pathology is seen. During this phase, damage occurs to the cardiovascular system, in the form of myocarditis or congestive heart failure, or to the digestive system, as megaesophagus or megacolon (megaviscera), in which the initial or terminal sections of the digestive system greatly enlarge and become dysfunctional, inhibiting the passage of food through the gut. These changes are secondary to injury to nervous tissue innervating the heart and digestive musculature. The muscles lose tone and become flabby, and the associated structures enlarge and lose functional capacity. Among persons who progress to the chronic phase, average life expectancy is reduced by nine years. In some regions of South and Central America, 10% of the deaths among adults may result from chronic Chagas’ disease.
In chronic Chagas’ disease, it is estimated that 4% will develop cardiomyopathy with heart failure; 18%, cardiomyopathy without heart failure; and 3%, megaviscera. Symptoms of cardiovascular disease in the chronic phase include tachycardia and arrhythmia. This progresses to cardiomyopathy with or without congestive heart failure, right bundle branch blockage, apical aneurysm, and thromboembolism, followed by death within two to three years. The first symptom of the digestive form of the chronic phase may be difficulty in swallowing, which may lead to decreased food intake and malnutrition. This is followed by the development of megaesophagus and megacolon as the musculature weakens and the lumens enlarge. Megaesophagus may be responsible for the difficulty in swallowing, while megacolon may lead to constipation and abdominal pain. This manifestation occurs primarily in southern Brazil and Argentina.
Inflammation and apoptosis, a self-destructive form of cellular death, may underlie some of the pathology found in Chagas’ disease. Parasite molecules attach to a toll-like receptor (TLR) on macrophages. TLRs serve as phagocyte receptors for recognition of molecular patterns of pathogens. Triggering of TLR2 stimulates macrophages to first secrete proinflammatory mediators, such as IL-12, TNF-α, and nitric oxide, and later induces a state of tolerance that may aid in long-term parasite persistence in the host. Cardiac damage may also occur at least in part from parasite-induced apoptosis of cardiomyocytes in inflammatory heart infiltrates during experimental Chagas’ disease. Lymphocyte apoptosis also increases replication of T. cruzi in macrophages in the vicinity, and blocking apoptosis reduces the number of parasites in the blood.
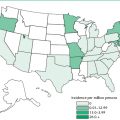
Trypanosoma cruzi and Chagas’ Disease in the United States
T. cruzi has been reported in appropriate insect vectors in several locations in the United States, including Alabama, Florida, Georgia, and Tennessee. Dogs and wild mammals, such as opossums and raccoons, are infected with T. cruzi in areas such as Texas, Oklahoma, Florida, Georgia, South Carolina, Virginia, California, and Louisiana. Infected dogs have abnormal electrocardiograms, arrhythmias, and signs of right-sided cardiac disease; half of those identified survive less than six months. In one study, 3.6% of the dogs were infected with the parasite. Veterinarians need to be aware of the possibility of canine Chagas’ disease among domestic animals. Infection of wild animals in the southern United States must also monitored because these animals may serve as reservoirs for the infection of humans and their pets. The infection rate among wild opossums in some areas was 37% and up to 61% for raccoons.
Human infection with T. cruzi or development of Chagas’ disease is being detected with ever greater frequency in the United States in California, Georgia, North Carolina, Tennessee, and Florida. In Los Angeles County, California, the proportion of infected blood samples containing antibodies to the parasite increased from 102 per 100,000 in 1996 to 185 per 100,000 in 1998. To assess the risk of transmission through blood transfusion in that region, donated blood was screened for antibodies to the parasite. Donors of infected blood were found to be 3.6 times more likely to have resided in Central America or Mexico and 8.7 times more likely to have previously donated blood. Infected organs donated for transplantation have been detected in California but not in a study of organs in the American Midwest.
In some cases, Americans who have no risk factors (travel to known endemic areas, blood transfusion, and drug usage) have developed Chagas’ disease. Animals in the vicinity of these individuals were found to be infected, suggesting that this disease may also be endemic to parts of the southern United States. Analysis of enzyme profiles of T. cruzi isolated from wild animals in the United States suggests that this parasite in indigenous to the country and has not entered the country in the recent past.
The Protozoan
Chagas’ disease is caused by the bloodborne parasite Trypanosoma cruzi. T. cruzi is a hemoflagellated protozoan of the class Zoomastigophera, order Kinetoplastida, family Trypanosomatidae, section Stercoraria. This section of protozoa develops in the posterior portions of the insect gut and is transmitted through fecal material expelled as the insect feeds. T. cruzi has been subdivided into zymodemes based on isoenzyme analysis. The Z1 zymodeme occurs in opossums and in domestic and peridomestic animals; Z2 is found in cats, dogs, domestic rodents, and guinea pigs; and Z3 is found primarily in opossums, with human infections uncommon. Trypanosoma brucei gambesea and T. b. rhodesiensence are relatives of T. cruzi that also cause human disease—African trypanosomiasis (African sleeping sickness). These parasites are from the section Salivaria and develop in the anterior gut with transmission via the saliva of tsetse bugs during biting. Other human pathogens related to T. cruzi include the Leishmania species, which cause cutaneous, mucocutaneous, and visceral leishmaniasis. The latter two conditions are extremely serious and often fatal.
T. cruzi assumes several morphologically and antigenically distinct forms throughout its life cycle. The metacyclic trypomastigote is the infectious form for humans. It is elongate and laterally flattened with the flagellum running along most of the length of its body, attached to the posterior end of the trypomastigote by an undulating membrane and extending beyond the parasite at its anterior end as a free flagellum. Members of the Trypanosomatidae family contain two distinct, dark-staining round bodies, the elongated central nucleus and the smaller kinetoplast. The latter is in close proximity to the origin of the flagellum in the basal body. It is posterior to the nucleus in the trypomastigote form, and its position relative the anterior of the parasite determines the length of the flagellum. The kinetoplast is a capsular extension of the single, large mitochondrion found in these parasites. It contains a high concentration of mitochondrial DNA existing in both linear and circular forms. A similar stage of the parasite found in the human bloodstream is the bloodstream trypomastigote, which looks much like the metacyclic trypomastigote, with a large round subterminal kinetoplast, but is more slender. It is the infectious form for the insect host. The bloodstream trypomastigote of T. cruzi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree