16 Central nervous system tumours
Introduction
Primary central nervous system (CNS) tumours can arise from any structures in the cranial vault. Around 4000 new patients with malignant brain tumours are diagnosed per year in the UK and 22,000 new patients in USA. There is a bimodal distribution with small peak in children (p. 323) and a steady increase starting at the age of 20 years. Males are more commonly affected, particularly with malignant tumours, whereas women have a higher rate of non-malignant tumours, particularly meningiomas.
Aetiology
Pathology
Box 16.1 shows WHO classification of brain tumours. WHO grading system (Box 16.2) is important in deciding management and prognosis. Molecular features can be incorporated in this grading system to yield important prognostic information. Clinico-pathological features of individual tumours are discussed later.
Box 16.1
WHO classification of brain tumours
Box 16.2
WHO grading of primary CNS tumours
Investigations
Imaging
CT scan
Contrast enhanced CT scan is usually the initial investigation in patients suspected to have a brain lesion. CT scan is not an ideal investigation for low-grade tumours or tumours in the posterior fossa. CT scan may also show features of oedema, hydrocephalus, haemorrhage and calcification depending on the histological variant of brain tumour. Table 16.1 shows radiological appearance of common tumours.
Table 16.1 Radiological appearance of common brain tumours
| Type of tumour | Imaging characteristic |
|---|---|
| Pilocystic astrocytoma | Well-circumscribed, contrast enhancing tumour with a cystic or enhancing mural nodule. |
| Grade II astrocytoma | Isodense or hypodense on CT. Hypointense on T1W image and hyperintense on T2W and FLAIR images. No contrast enhancement and if present suggest malignant transformation. No associated cerebral oedema. |
| Oligodendroglioma | Same CT/MRI as grade II astrocytoma; but can be associated with areas of contrast enhancement, calcification and haemorrhage. |
| Anaplastic astrocytoma and GBM | CT – irregular hypodense lesions with varying degree of contrast enhancement and associated oedema and pressure effect. Ring-like enhancement surrounding an irregular shaped necrosis suggests GBM. Some of the high-grade tumours can be non-enhancing particularly in the elderly. |
| Anaplastic oligodendroglioma | Contrast enhancing heterogeneous mass with frequent cystic components, calcifications and necrosis. |
| Medulloblastoma | CT – hyperdense midline mass with associated hydrocephalus. Marked contrast enhancement. Sometimes show calcification cysts, haemorrhage and nodular seedling. |
| Ependymoma | Heterogeneously enhancing lesion with cystic component. There may be associated calcification and haemorrhage. |
| Primary CNS lymphoma | Solitary or multiple periventricular homogenously enhancing diffuse lesions (‘cotton wool’ appearance). Ring enhancement common in immunocompromised patients. |
| Meningioma | Homogenously contrast enhancing lesion arising from the dura. There is little associated brain oedema. There will be enhancement of dura (’dural tail’ sign). |
| Craniopharyngioma | Solid and cystic lesion on CT scan. MRI appearance depends on the composition of tumour. T1W can be hypo, iso or hyperintense with variable contrast enhancement. T2W can also be hypo, iso or hyperintense. |
| Pituitary | Contrast enhancing seller/suprasellar lesion on CT. T1W iso-hypointense and T2W hyperintense. |
| Metastasis | Solitary or multiple irregularly contrast enhancing lesion which may be solid or cystic at the junction of grey and white matter. Location and characteristic depends on type of tumour. |
MRI scan
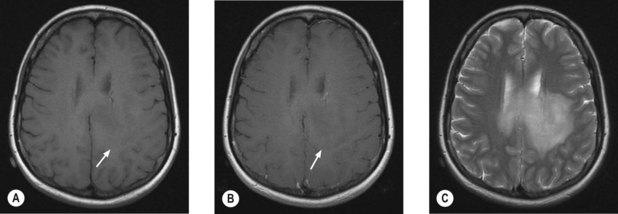
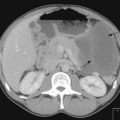
MRI scan with gadolinium and FLAIR (fluid-attenuated inversion recovery) sequence is the standard investigation for brain tumours. T1W imaging demonstrates anatomy and areas of contrast enhancement and T2W and FLAIR images are useful in demonstrating oedema. Appearance of tumour on T1W image is similar to that on CT, but better delineated on MRI. Tumour and oedema demonstrate increased signal on T2 and the area of increased T2 signal on MRI usually includes the hypodense area on CT (Figure 16.1).
Diffusion tensor imaging (DTI)
In radiotherapy planning for high-grade gliomas, conventional methods of imaging cannot distinguish oedema from peritumoural white matter infiltration, resulting in large target volumes. DTI shows white matter abnormalities resulting from tumour infiltrating, and thereby reducing the target volume, which would allow significant dose escalation to the tumour with acceptable damage to the normal tissue.
Evaluation of the craniospinal axis
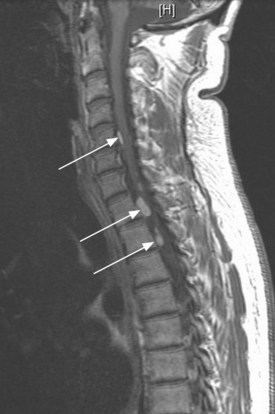
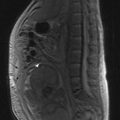
MRI of the craniospinal axis and CSF evaluation is needed for tumours with a high risk of CSF dissemination such as medulloblastoma, germ cell tumours, CNS lymphoma, PNET and ependymoma (Figure 16.2). CSF evaluation includes CSF biochemistry (typically elevated protein >40 mg/dL and reduced glucose <50 mg/mL), cytology and in cases of suspected germ cell tumours, the estimation of tumour markers (AFP and beta hCG) in CSF and serum. Evaluation of the craniospinal axis is best done prior to surgery or >3 weeks after surgery to avoid false positive results.
Prognostic factors
The outcome of brain tumours depends on the following factors:
Principles of management
General medical management
Steroids
Steroids may improve symptoms by reducing intracranial pressure. The most commonly used agent is dexamethasone which is started at a dose of 2–16 mg daily (low doses given once daily and higher doses as 2–4 equally divided doses) and titrated against the patient’s symptoms. The optimal dose is just above that at which symptomatic deterioration occurs. If there is no symptomatic improvement, steroids should be stopped.
Surgical management
Surgery in brain tumours helps in pathological diagnosis, symptom control and definite treatment.
Radiotherapy in brain tumours
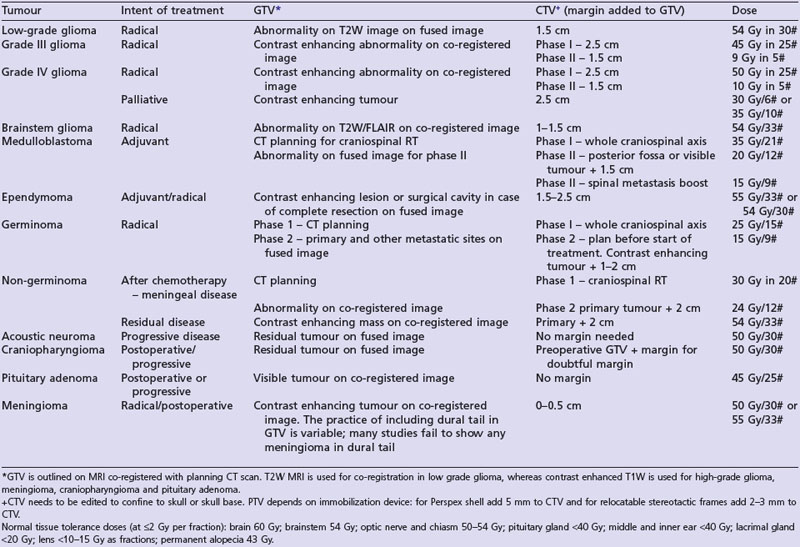
A summary of radiotherapy details for common tumours are given in Table 16.2.
Radiation tolerance and reactions
Acute radiation reactions may manifest up to 6 weeks following completion of irradiation. These reactions are generally those of raised intracranial pressure, fatigue and worsening of neurology. Vomiting is particularly common in patients receiving radiotherapy to the brainstem region.
Delayed radiation reaction develops 6 months after radiotherapy. This is thought to be due to white matter injury secondary to vascular injury, demyelination and necrosis. The most serious form of this is radiation necrosis which peaks at 3 years. Conventional imaging fails to distinguish this from recurrent tumour and special imaging is therefore needed (p. 265). Treatment is with steroids and debulking. Other late effects include, depending on the area irradiated, memory problems, pituitary failure, hearing loss, visual changes and second malignancies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree