Central Nervous System Infections
Jeffrey M. Tessier
W. Michael Scheld
Healthcare-associated infections (HAIs) of the central nervous system (CNS) are a rare but serious occurrence in the modern hospital. As with other types of HAIs, infection of the CNS most often follows a procedure that provides access for microbes to bypass normal host barriers. While a majority of episodes follow neurosurgery (NS), other neuroinvasive procedures (e.g., lumbar puncture or placement of an epidural catheter) can occasionally lead to infection of the CNS (1,2). Healthcare-associated CNS infections that are not due to microbial contamination during procedures generally affect immunosuppressed patients or neonates who possess an immature blood-brain barrier that may be more easily crossed during bacteremia. Healthcare-associated CNS infections range from superficial surgical site infections (SSIs) as the result of neurosurgery to meningitis, meningoencephalitis, or focal suppurations including brain abscess, subdural empyema, or epidural abscess. A recent review of healthcare-associated bacterial meningitis provides the reader with an excellent additional reference to this chapter (3).
INCIDENCE
Data from the Centers for Disease Control and Prevention’s (CDC) National Nosocomial Infections Surveillance (NNIS) program, which were collected in 163 U.S. hospitals between 1986 and 1993, document 5.6 NS healthcare-associated CNS infections for every 100,000 patients discharged (4). This rate is approximately half of what it was a quarter of a century ago (1/10,000 discharges) (5). Meningitis is the most common CNS infection, accounting for 91% of the total, followed by intracranial abscesses in 8% and spinal abscesses in only 1%.
Somewhat higher rates of CNS infection have been observed among the immunosuppressed, ranging from 20 per 100,000 discharges for cancer patients (6,7) to 2.5 per 100 among heart transplant patients (8). Meningitis has constituted 71% of CNS infections among cancer patients, followed by brain abscess and encephalitis (making up 27% and 2% of episodes, respectively) (6). Brain abscess appears to be more common among transplant patients (see Chapter 45), accounting for ~40% of CNS infections after heart and heart-lung transplants (9). The highest rate of infection for a hospital service has been 45 per 100,000 discharges for the newborn nursery, according to NNIS data collected between 1986 and 1990 (10).
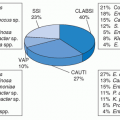
The incidence of CNS infection is relatively high among NS patients compared with other groups of patients. Among patients with American Society of Anesthesiology (ASA) scores <3, an operative duration <75th percentile, and a wound classification of “clean” or “clean contaminated,” infection rates in the NNIS program have been 0.56/100 craniotomies, 0.70/100 spinal fusions, and 3.85/100 ventricular shunts (11). From 2006 to 2008, as per CDC’s National Healthcare Safety Network (NHSN) report, pooled mean SSI rates for spinal fusions of risk index 0 was 0.70 (median 0.74), risk index 1.0 was 1.84 (median 1.70), and risk index of 2.0 or 3.0 was 4.15 (median 3.35) (http://www.cdc.gov/nhsn/PDFs/dataStat/2009NHSNReport.PDF). Among patients with higher ASA scores (i.e., greater severity of underlying illnesses), longer durations of the procedure, and/or contamination of the wound, higher rates were observed (11). The most common infection following NS procedures has been superficial SSI, accounting for 60% of SSIs after craniotomy and 75% after laminectomy, according to NNIS data (4). According to updated NNIS data from January 1992 through June 2004, the rate of SSI after craniotomy was 2.40/100 operations when patients had a risk index category of 2 or 3. This rate was reduced to 1.72/100 operations and 0.91/100 operations when the risk index category was 1 or 0, respectively. From 2006 to 2008, the craniotomy SSI pooled mean rate for risk index 0 or 1.0 was 2.15 (median, 1.51) and for risk index 2.0 or 3.0 was 4.66 (http://www.cdc.gov/nhsn/PDFs/dataStat/2009NHSNReport.PDF). Ventricular shunt placement resulted in SSI in 5.35/100 operations with a risk factor index of 1 to 3 compared to 4.42/100 operations with a risk factor index of 0 (12). From 2006 to 2008, for ventricular shunts, the pooled mean SSI rate was 4.04 for risk index 0 patients and 5.93 for risk index 1.0, 2.0, or 3.0 patients (http://www.cdc.gov/nhsn/PDFs/dataStat/2009NHSNReport.PDF). Meningitis is the second most common CNS infection after craniotomy, accounting for 22% of episodes, and it is the most common form of CNS infection after ventricular shunt placement, accounting for 76% of episodes.
RISK FACTORS
The most obvious risk factor for healthcare-associated CNS infection has been NS. Skin flora, which usually cannot be cultured from the operative site immediately after antiseptic preparation, regrow during the operation and can be cultured from a majority of operative sites just before closure (13). As with other types of surgery, it is thus likely that most infections occur during the procedure while the wound is open, becoming contaminated from regrowth of the patient’s own skin flora at the margins of the wound or occasionally by organisms from the operative team introduced on contaminated gloves or instruments or settling from the air into the wound. Infection at another body site also is a risk factor for infection of the NS wound. In an outbreak of meningitis due to Klebsiella spp., for example, colonization and/or infection of the respiratory or urinary tracts appeared to precede CNS
infection (14). Another study found that in 70% of NS patients with meningitis, there was antecedent or simultaneous isolation of the same organism from another body site (15). A review of 15,200 NS procedures performed at one tertiary care center from January 1986 to December 2001 revealed an infection rate of 0.28% (35/12,980) after craniotomy and 1.20% (27/2,220) after ventriculostomy or ventriculoperitoneal shunt insertion, with an overall infection rate of 0.40% (16). Another comprehensive review of 51,133 patients admitted to an NS service from 1993 to 2002 revealed 51 episodes of healthcare-associated meningitis, all of which were associated with NS intervention. Ventriculoperitoneal shunt procedures, either insertion or revision, accounted for 26% of the episodes. The next largest group consisted of patients undergoing surgery for an intracranial mass (17). A total of 74% of bacterial meningitis in 61 patients aged 17 to 40 years identified in Taiwan had a postneurosurgical state as an underlying condition (18).
infection (14). Another study found that in 70% of NS patients with meningitis, there was antecedent or simultaneous isolation of the same organism from another body site (15). A review of 15,200 NS procedures performed at one tertiary care center from January 1986 to December 2001 revealed an infection rate of 0.28% (35/12,980) after craniotomy and 1.20% (27/2,220) after ventriculostomy or ventriculoperitoneal shunt insertion, with an overall infection rate of 0.40% (16). Another comprehensive review of 51,133 patients admitted to an NS service from 1993 to 2002 revealed 51 episodes of healthcare-associated meningitis, all of which were associated with NS intervention. Ventriculoperitoneal shunt procedures, either insertion or revision, accounted for 26% of the episodes. The next largest group consisted of patients undergoing surgery for an intracranial mass (17). A total of 74% of bacterial meningitis in 61 patients aged 17 to 40 years identified in Taiwan had a postneurosurgical state as an underlying condition (18).
The factors that amplify the risk of postcraniotomy infection have included duration of the operation, external drainage, re-exploration, and operation through a paranasal sinus (19). The risk factors contributing to the development of meningitis/ventriculitis after placement of a ventriculostomy catheter have included intracerebral hemorrhage, other NS operations, drainage for >5 days, an air-vented system, irrigation of the system, and intracranial pressure >20 mm Hg (19,20). The risk for infection of cerebrospinal fluid (CSF) shunts is increased by the duration of the procedure, thrombosis of the catheter, externalization of the shunt, inexperience on the part of the surgeon, and type of shunt (ventriculoatrial carrying a higher risk than ventriculoperitoneal shunting) (19). A persistent CSF leak after surgery heightened the risk of infection 13-fold in one study (21); concurrent infection at a remote site increased the risk of CNS infection 6-fold.
Korinek et al. prospectively evaluated every adult patient undergoing craniotomy in 10 NS units during a 15-month period. Of the 2,944 patients studied, 117 patients developed SSIs. Independent SSI risk factors were postoperative CSF leakage (odds ratio [OR], 145; 95% confidence interval [CI], 72 to 293) and subsequent operation (OR, 7; 95% CI, 4 to 12). Independent predictive risk factors were emergency surgery, clean-contaminated and dirty surgery, an operative time >4 hours, and recent NS. Absence of antibiotic prophylaxis was not a risk factor. The investigators also found that the NNIS risk index was effective in identifying at-risk patients (22).
Placement of an intracranial pressure monitor is associated with different rates of infection, depending on where the monitor is positioned. One study found a 7.5% infection rate with a subarachnoid screw, a 14.9% rate with a subdural cup catheter, and a 21.9% rate for a ventriculostomy catheter (23). Another study found a 0.6% rate for epidural monitors, a 3.0% rate for a subdural bolt, and a 4.0% rate for intraventricular or parenchymal brain monitors (24).
In June 2002, a manufacturer of cochlear implants used to enhance the perception of sounds in patients with severe to profound hearing loss notified the Food and Drug Administration (FDA) of 15 reports of postimplantation bacterial meningitis in patients who received these implants. This led to a cohort study to determine the incidence of bacterial meningitis among children with cochlear implants and a nested case-control study to examine the risk factors for meningitis. The incidence of all episodes of meningitis in the cohort was 239.3/100,000 person-years (95% CI, 156.4 to 350.6). Perioperative meningitis occurred at a rate of 2.1 episodes per 1,000 procedures. On multivariate modeling, the use of a positioner was significantly associated with meningitis (OR, 4.5; 95% CI, 1.3 to 17.9), as was inner-ear malformation with a CSF leak (OR, 9.3; 95% CI, 1.2 to 94.5) (25).
Gliadel® wafers are approved for the treatment of malignant gliomas. These dime-sized disks contain carmustine (1,3-bis(2-chloroethyl)-1-nitrosourea), the primary chemotherapeutic agent used to treat glioblastoma multiforme. Initial studies reported an SSI rate of <5% with wafer insertion, but subsequent reports revealed infection rates of 15% to 23%. A 2003 review of 32 patients who received a Gliadel® wafer identified nine patients who developed an SSI. Among these nine patients, there were four episodes of brain abscess, four of bone flap osteitis, two of epidural abscess, and one each of cellulitis and subgaleal abscess associated with wafer insertion (26).
Patients with head trauma are at increased risk of CNS infection, especially meningitis. CSF fistula raises the risk of infection in this population. A CSF leak was found to be present in 13% of episodes of healthcare-associated meningitis after head trauma in one series (27). Infection of the paranasal sinuses may be followed by CNS infection in these patients (28).
A rare but important risk factor for healthcare-associated bacterial meningitis to consider is the diagnosis of acute bacterial meningitis (ABM) itself. This phenomenon of “super-infection” has been studied by Huang et al., who reported 21 patients with 27 episodes of healthcare-associated meningitis after being diagnosed with ABM over a 9.5-year period in Taiwan (29). Superinfection was identified in this study as growth of a new pathogen from the CSF during the therapeutic course of existing meningitis. All 21 patients underwent a neurosurgical procedure/device (External ventricular drains (EVD), VP shunt, or Ommaya reservoir) temporally associated with the superinfection. Recurrent fever was the most common clinical finding, and the recovered CSF pathogens implicated in the superinfections were predominantly drug-resistant gram-negative bacilli. Superinfections were associated with a high mortality rate (33.3%).
Premature birth also appears to be an important risk factor for CNS infection because neonates cared for in neonatal intensive care units (NICUs) have had the highest rates of CNS infection according to NNIS data (10). This finding seems to be related to the high risk of bacteremia from critical care instrumentation coupled with an increased risk of secondary meningitis from bacteremia stemming from the neonate’s immature blood-brain barrier. Immunosuppression is another important risk factor for nonsurgical CNS infection, usually due to hematogenous spread.
ETIOLOGIC AGENTS
Staphylococci and gram-negative bacilli accounted for almost 70% of CNS infections documented in NNIS hospitals between 1986 and 1992 (4). During this time, Staphylococcus aureus was the most common pathogen after both craniotomy and laminectomy, followed by coagulase-negative staphylococci. These organisms were followed by enterococci, Streptococcus spp., Pseudomonas aeruginosa, Acinetobacter spp., Citrobacter spp., Enterobacter spp., Klebsiella pneumoniae, Escherichia coli, miscellaneous other gram-negative bacilli, and yeast, each of which accounted for <10% of episodes. After shunt procedures, S. aureus
remained the most common pathogen causing superficial SSIs, but coagulase-negative staphylococci were more typical causes of deeper SSIs; gram-negative bacilli were responsible for 19% of deeper SSIs related to shunts (4). Streptococcus pneumoniae was the predominant pathogen in the cohort of children with bacterial meningitis as a complication of cochlear implants (15/24), and Staphylococcus spp. were predominantly isolated after infectious complications of Gliadel® wafer insertion (25,26).
remained the most common pathogen causing superficial SSIs, but coagulase-negative staphylococci were more typical causes of deeper SSIs; gram-negative bacilli were responsible for 19% of deeper SSIs related to shunts (4). Streptococcus pneumoniae was the predominant pathogen in the cohort of children with bacterial meningitis as a complication of cochlear implants (15/24), and Staphylococcus spp. were predominantly isolated after infectious complications of Gliadel® wafer insertion (25,26).
If all CNS infections are considered, coagulase-negative staphylococci were the most frequent pathogens, making up 31% of episodes compared with 27% for gram-negative bacilli, 11% for Staphylococcus aureus, 18% for Streptococcus spp., 4% for yeast, and 9% for others (4). For meningitis, the most frequently encountered CNS infection, coagulase-negative staphylococci accounted for 32%, followed by gram-negative bacilli (29%), Streptococcus spp. (18%), S. aureus (10%), yeast (4%), and others (9%) (4). For intracranial infections, gram-negative bacilli were the cause of 23% of episodes, followed by S. aureus (19%), coagulase-negative staphylococci (17%), anaerobes (11%), fungi (8%), Streptococcus spp. (8%), viruses (4%), yeast (3%), and others (8%). Spinal abscess displayed a dramatically different distribution of etiologic agents: 67% were due to S. aureus and 33% were due to coagulase-negative staphylococci (4).
The largest study of healthcare-associated meningitis from a single hospital was conducted by Durand et al., who reviewed 197 episodes among 151 adult patients at Massachusetts General Hospital during a 27-year period (27). These healthcare-associated episodes accounted for 40% of the total of 493 episodes of bacterial meningitis observed during the study period. The proportion of episodes that were HAIs increased during the 27-year period. In this study, gram-negative bacilli were most common, accounting for 38% of episodes, followed by S. aureus (9%), coagulase-negative staphylococci (9%), Streptococcus spp. (9%), Haemophilus influenzae (4%), Listeria monocytogenes (3%), and Enterococcus spp. (3%) (27). The microbes responsible for healthcare-associated gram-negative meningitis in this study were E. coli (30%), Klebsiella (23%), Pseudomonas (11%), Acinetobacter (11%), Enterobacter (9%), Serratia (9%), Citrobacter (4%), Proteus (2%), coliform types (2%), and nonenteric types (2%) (27). The higher proportion of gram-negative and lower proportion of staphylococcal isolates in this study than in the more recent NNIS data could be due to the fact that there were only 2 years of overlap between the 27-year study and the NNIS data.
In Wang’s review of 15,200 operative NS procedures from 1986 to 2001, the most frequently isolated pathogen was S. aureus (13/62, 21%); 91% of episodes involved a single pathogen with coagulase-negative Staphylococcus (7/62, 11%), P. aeruginosa (5/62, 8%), E. coli (5/62, 8%), and Acinetobacter baumannii (4/62, 6%) following S. aureus in frequency (16). A review of S. aureus CNS infections in Denmark over a 16-year period (1984 to 1999) identified 45 episodes of meningitis and 5 episodes of brain abscess. Forty-four of these episodes were HAIs, and only six were community-acquired. None of the isolates was methicillin- resistant, and six were penicillin-susceptible (30).
Methicillin-resistant Staphylococcus aureus (MRSA) now represents a substantial percentage of S. aureus isolates in most hospitals in the United States and other countries around the world. A recent multi-institutional study from Spain examined 86 episodes of MRSA meningitis, with 80 of these being identified as healthcare-associated (93%) (31). The vast majority of healthcare-associated episodes (78/80, 97.5%) were postoperative complications, mainly associated with CSF devices (74%), neurosurgery (45%), CSF leaks (17%), and head trauma (12%). MRSA meningitis in this study carried a 30-day mortality of 31%, and multivariate analysis identified spontaneous origin (i.e., not identified as healthcare-associated in origin; OR, 21.4; 95% CI, 2.3 to 195.4; p = .007) and development of coma (OR, 9.7; 95% CI, 2.2 to 42.3; p = .002) as independent predictors of death.
A. baumannii has become a more commonly reported pathogen in episodes of healthcare-associated meningitis throughout the world, a phenomenon attributed to the use of broad-spectrum antimicrobials, especially carbapenems, and the emergence of carbapenem-resistant A. baumannii strains. The mortality of A. baumannii meningitis (ABM) reported in published studies is high, in the range of 30% to 70%, depending on the report (32,33,34), and appears related to the antimicrobial activity of empiric drug selection.
Candida spp. are infrequently implicated as causative agents in healthcare-associated CNS HAIs. O’Brien et al. performed a retrospective, single-center study of Candida CNS infections after NS over an 11-year period (1998 to 2009) (35). They identified 11 episodes via laboratory review of positive cultures from CNS specimens. Candida albicans was the most commonly identified yeast (73%), and all of the episodes were associated with the presence of foreign material in the CNS (nine EVD, one VP shunt, one lumbar drain, one Gliadel® wafer).
Although large outbreaks of fungal meningitis are very rare, in 2012 to 2013, there was a very large outbreak of fungal meningitis and spinal epidural abscesses traced to epidural or paraspinal glucocorticoid injections of intrinsically contaminated preservative-free methylprednisolone acetate prepared by a single compounding pharmacy (36).
Pathogens most frequently isolated from brain abscesses have traditionally been streptococci, Enterobacteriaceae, and anaerobes. However, a recent prospective study of bacteria associated with 20 brain abscesses in Marseilles, France, utilized 16S rRNA sequencing to identify fastidious organisms (37). This study found an extremely broad array of bacteria in brain abscesses, including several new organisms not previously described, and confirmed the polymicrobial nature of these infections. Conventional culture techniques identified a total of 22 species of bacteria in this study, whereas multiple sequencing of 16S rRNA identified 72 species. Importantly, Mycoplasma spp. were commonly identified in brain abscesses (25% of specimens); this genus has not traditionally been considered when designing empiric antimicrobial regimens to treat brain abscesses. Among immunocompromised patients, the frequency distribution of etiologic agents is somewhat different; Toxoplasma gondii and Cryptococcus neoformans are most frequent in patients with the acquired immunodeficiency syndrome, Aspergillus spp. and T. gondii are the most common after heart and heart-lung transplants, and Aspergillus spp. and C. neoformans are the most typical after kidney and liver transplants (38). Among bone marrow transplant recipients, fungi accounted for 92% of episodes in a comprehensive study: Aspergillus spp. in 58% of episodes and Candida spp. in 33% (38). A South African, single-center, retrospective study of brain abscesses requiring surgical intervention (N = 121) found Nocardia spp. in 2.5% of patients (39); these organisms are well-described causes of community-acquired infections in immunocompromised patients. A retrospective hospital-based epidemiologic study identified 153 patients with brain abscess over a 15-year period (1986 to 2000). There were 103 community-acquired
infections and 20 HAIs. Of the HAIs, 17 occurred in a post-neurosurgical state. Overall, K. pneumoniae and viridans streptococci were the two most prevalent pathogens, and the addition of S. aureus accounted for 47% of post-NS brain abscesses (40).
infections and 20 HAIs. Of the HAIs, 17 occurred in a post-neurosurgical state. Overall, K. pneumoniae and viridans streptococci were the two most prevalent pathogens, and the addition of S. aureus accounted for 47% of post-NS brain abscesses (40).
Outbreaks of CNS infection among neonates or in NS patients most often have involved aerobic gram-negative bacilli (12,41,42,43,44,45). Such outbreaks sometimes have been linked to a healthcare provider carrying the organism (46) and at other times to contaminated equipment, such as respirators (47) or a shaving brush used for preoperative hair removal (45). A review of 30 adult patients with gram-negative bacillary meningitis found that the majority of episodes occurred in men and that E. coli was isolated most frequently (48). Outbreaks due to gram-positive bacteria, such as Streptococcus spp. (both groups A and B), S. aureus, and L. monocytogenes, also have been reported (49,50,51,52,53,54




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree