Hematologic disorders affect the central nervous system in a variety of ways, producing a wide range of neurologic disturbances. Early identification of these complications allows for early intervention and better outcome. Cross-sectional imaging plays an important role in identifying brain abnormalities and helps the clinician in deciding appropriate course of action and treatment. This article discuss in short the basics of hemostasis including the coagulation cascade and the application of basic laboratory tests in evaluation of hematologic function. Imaging features of various neurologic disorders associated with these clotting and bleeding diatheses are discussed in detail with illustrations.
Key points
- •
Hematologic disorders can affect the central nervous system in a variety of ways, producing wide range of neurologic disturbances.
- •
It is important to identify these complications as early as possible for early intervention and better outcome.
- •
Cross-sectional imaging, mainly CT and MRI, plays an important role in identifying brain abnormalities and thus helps the clinician in deciding the appropriate course of action and treatment.
Introduction
Hematologic disorders can affect the central nervous system (CNS) in a variety of ways, producing a wide range of neurologic disturbances. It is important to identify these complications as early as possible for early intervention and better outcome. If untreated some of these complications may have irreversible deficit to fatal outcome. Today cross-sectional imaging, mainly computed tomography (CT) and MRI, plays an important role in identifying brain abnormalities and thus helps the clinician in deciding the appropriate course of action and treatment. This article reviews the basics of hemostasis including the coagulation cascade and the application of basic laboratory evaluation of hematologic function. The standard classification of the hematologic disorders is used to categorize the clotting and bleeding diatheses. The imaging features of various neurologic disorders associated with these clotting and bleeding diatheses are then discussed in detail.
Introduction
Hematologic disorders can affect the central nervous system (CNS) in a variety of ways, producing a wide range of neurologic disturbances. It is important to identify these complications as early as possible for early intervention and better outcome. If untreated some of these complications may have irreversible deficit to fatal outcome. Today cross-sectional imaging, mainly computed tomography (CT) and MRI, plays an important role in identifying brain abnormalities and thus helps the clinician in deciding the appropriate course of action and treatment. This article reviews the basics of hemostasis including the coagulation cascade and the application of basic laboratory evaluation of hematologic function. The standard classification of the hematologic disorders is used to categorize the clotting and bleeding diatheses. The imaging features of various neurologic disorders associated with these clotting and bleeding diatheses are then discussed in detail.
Coagulation cascade
Normal hemostasis is an elaborate, multifactorial process involving the interaction of multiple elements to produce two main physiologic effects: the maintenance of liquidity of blood and prevention of bleeding after vessel damage. Normal platelet function maintains hemostasis in an undamaged vessel. Active hemostasis, or formation of fibrin in blood vessels, depends on progression through three main processes: (1) vascular, (2) platelet, and (3) coagulation stages of clot formation. The vessel and vascular endothelium play an important primary role in the initiation of clotting. Vascular constriction at the time of injury can be the sole mechanism of preventing minor bleeding. In addition, vascular endothelium damage exposes blood to collagen, fibrinogen, and von Willebrand factor (vWF), which stimulates platelet adhesion. Conversely, intact endothelium is responsible for inhibition of hemostasis through prostacyclin and nitrogen oxide, which inhibit platelet aggregation.
Platelet aggregation contributes to the next phase of hemostasis by binding circulating vWF and collagen released from injured endothelium to its surface receptors. After aggregation and activation, platelets undergo morphologic change forming a plug that releases substances, such as calcium, serotonin, and ADP, which promote further coagulation. Many additional factors, which play a role in coagulation, are released from dense granules secreted by aggregating platelets. These factors include platelet factor 3; β-thromboglobulin; platelet-derived growth factor; thrombospondin; factor V; and plasma proteins, such as fibrinogen and IgG.
The final stage of the clotting process is the coagulation cascade, where a series of coagulation factors become sequentially activated to produce a fibrin clot ( Fig. 1 ). There are two coagulation pathways, which culminate into a final common pathway. The liver produces factors involved in these pathways, therefore liver disease predisposes to dysfunction of the coagulation cascade. The intrinsic coagulation pathway begins with the activation of factor XII and conversion into factor XIIa; this activates the conversion of factor XI to XIa. Factor XIa activates the conversion of factor IX to IXa, which ultimately leads to the conversion of factor X to factor Xa, which is the initial step of the common pathway. The shorter, extrinsic pathway begins with the simultaneous activation of factor VII to VIIa and activation of factor III (tissue thromboplastin). Both of these factors cause the conversion of factor X to Xa. The activation of the common pathway begins with factor Xa, which activates the conversion of thrombin to prothrombin I. Prothrombin I in turn activates the conversion factor XIII to XIIIa. At the same time, thrombin activates polymerization of fibrinogen to fibrin polymers, which are converted to a fibrin meshwork making up the clot by the action of factor XIIIa.
Laboratory evaluation
The disturbance of these pathways, and alteration in production or function of coagulation factors, are responsible for producing the spectrum of clotting and bleeding disorders. Several basic laboratory tests help in their distinction:
- •
Bleeding time: an older test, now mostly replaced by quantitative tests that measure platelet function under conditions of high sheer stress. Bleeding time test was used in the past to assess in vivo response to vascular injury by measuring a standard time for an induced skin puncture to stop bleeding.
- •
Platelet count: a test to quantify platelet availability by counting the number of platelets by an electronic counter in an anticoagulated blood sample. The count is then confirmed by visual inspection of a comparative blood smear. Disorders of platelet number, such as thrombocytopenia, are diagnosed by this method.
- •
Prothrombin time (PT): a test of the extrinsic and common coagulation pathways. After addition of calcium ion and tissue thromboplastin to a sample of plasma, time to coagulation is assessed. Prolonged coagulation time points to deficiency or dysfunction of factors V, VII, X, prothrombin, or fibrinogen.
- •
Partial thromboplastin time (PTT): similar to PT, this test evaluates the function of the intrinsic coagulation pathway. Clotting time of plasma is assessed after the addition of calcium ion with kaolin and cephalin. The function and amount of available factors V, VII, IX, X, XI, XII, prothrombin, or fibrinogen may be assessed.
In addition, various other tests may be performed to diagnose or classify the underlying pathology. Some of these tests may include peripheral smear, bone marrow biopsy, and organ biopsy. Discussion of these investigations is beyond the scope of this article.
Classification
This article is organized under two main headings: hemorrhagic and coagulation disorders. Hemorrhagic disorders may be caused by a disorder of one or more factors that participate in hemostasis. Most hemorrhagic syndromes are related to blood vessel disorders (vasculopathies), platelet number (thrombocytopenias) and function disorders (thrombocytopathies), or coagulopathies Table 1 . Coagulopathies are caused by the absolute absence or decrease of the synthesis of certain coagulation factors, or the production of abnormal coagulation factor molecules or increased destruction during intravascular coagulation, or by the presence of inhibitors. This deficiency may be inherited or, more frequently, may result from organ disorders participating in the formation of coagulation factors.
| Vasculopathies (functional abnormalities of vascular wall) | Hereditary | Hereditary hemorrhagic telangiectasia Hereditary disorders of connective tissue (eg, Ehlers-Danlos syndrome) |
| Secondary | Infections Chemical factors or drugs Disorders of metabolism (vitamin C or substance P deficiency) Pathologic changes of vascular wall (atherosclerosis) Connective tissue diseases | |
| Allergy: allergic purpura | Henoch-Schönlein purpura, purpura simplex, senile purpura, mechanic purpura, paraproteinemia | |
| Platelet disorders | Thrombocytopenia | See Table 2 |
| Thrombocytosis | Primary: essential thrombocythemia Secondary: infections, injury, postsplenectomy chronic myelocytic leukemia, myeloproliferative disorders (polycythemia vera) | |
| Functional abnormalities of platelets | Congenital: thrombasthenia, giant platelet syndrome Acquired: drugs, uremia, liver diseases, and dysproteinemias | |
| Coagulation disorders caused by coagulation factor deficiencies | Congenital | Hemophilia A (factor VIII deficiency) Hemophilia B (factor IX deficiency) Factor XI deficiency (hemophiliac), hypothrombinogenemia, hypofibrinogenemia Von Willebrand disease Hereditary thrombophilia: antithrombin deficiency, protein C deficiency, protein S deficiency, factor V Leiden (RQ506Q), prothrombin G:A 20210 mutation |
| Acquired | Vitamin K deficiency Severe liver diseases Drugs (dicumarol) Disseminated intravascular coagulation | |
| Hyperfibrinolysis | Primary | Congenital deficiency of α 2 -antiplasmin, clinical use of urokinase, liver diseases, liberation of tissue plasminogen activator into the circulation |
| Secondary | Disseminated intravascular coagulation |
Increased vessel fragility
Functional abnormality in the vessel wall may cause either macrohemorrhages or microhemorrhages in the various organs of the body. These hemorrhages are predominately seen as small dermal and mucous membrane petechia or purpura. It is not uncommon for these disorders to present with neurologic deficit caused by intracranial hemorrhage or infarction. They may be hereditary, such as hereditary hemorrhagic telangiectasia (HHT) or Ehlers-Danlos syndrome (EDS), a heritable disorder of connective tissue. Alternatively they may be secondary to infections, chemical factors, drugs, disorders of metabolism (vitamin C or substance P deficiency), pathologic changes of vascular wall (atherosclerosis), or connective tissue diseases. Vessel wall abnormality may also be seen with allergic conditions, such as purpura and Henoch-Schönlein purpura. These disorders usually have a normal bleeding time, platelet count, PT, and PTT.
Hereditary Causes
Hereditary hemorrhagic telangiectasia
HHT, also referred to as Osler-Weber-Rendu disease, is an autosomal-dominant disorder characterized by multiple arteriovenous malformations (AVMs). As a result the disorder presents with dilated, tortuous blood vessels with thin vessel walls that rupture and bleed easily. The diagnosis is usually not suspected until adolescence or later when patients present with frequent epistaxis secondary to rupture of small AVMs near the skin surfaces and mucous membranes.
HHT can present at any age, including infancy. CNS presentations in HHT may be caused by strokes usually secondary to pulmonary AVM/arteriovenous fistula (AVF), brain AVM with bleed, or subarachnoid hemorrhage from saccular aneurysm. In the brain parenchymal capillary telangiectasias are relatively rare compared with AVM. Disorder is thought to be caused by abnormal transforming growth factor-β signal transduction, which affects the vasculogenesis, angiogenesis, and endothelial cell properties. Diagnosis is often made by documenting multiple pulmonary AVM or cerebral AVM in a patient with recurrent epistaxis. There are several genes responsible for the disorder and diagnosis is made by clinical presentation followed by genetic testing including a search for pathologic variants of genes involved in the transforming growth factor-β/bone morphogenetic proteins (BMP) signaling cascade.
On an unenhanced CT of the brain AVM appears as isodense serpentine vessels, which shows intense uniform enhancement of vascular channels and nidus on contrast-enhancement scans. MRI evaluation shows cerebral AVMs as a tangle of vessels with flow voids and possible hemorrhages on T1- and T2-weighted images. T2-weighted sequences may also show edema, mass effect, and gliosis surrounding the AVMs ( Fig. 2 ). Additional large AVMs occur in the liver and lungs where they also present with bleeding or shunting complications. Treatment is based on symptoms, and in the brain, cerebral AVMs are treated surgically, embolized, or are treated with stereotactic radiosurgery. In diagnosed or suspected cases of HHT, screening for presence and evolution of cerebral AVMs is recommended with MRI in childhood (usually at the time of diagnosis) and again after puberty.
Ehlers-Danlos syndrome
Vessel wall fragility producing microvascular bleeds may be seen because of impaired formation of collagen that is needed for vessel wall support. Such disorders include EDS, scurvy, and Cushing syndrome.
EDS is a heritable connective tissue disorder characterized by skin hyperextensibility, fragile and soft skin, delayed wound healing with formation of atrophic scars, easy bruising, and generalized joint hypermobility. Fifty percent of patients with classic EDS harbor mutations in the COL5A1 and the COL5A2 gene, encoding the 1- and the 2-chain of type V collagen, respectively.
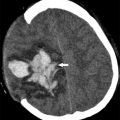
The diagnosis of EDS is established by clinical examination and family history. Cardiac lesions are common and include mitral and tricuspid valve prolapse, septal defects, and dilatation of the aortic root and pulmonary arteries. The most important and frequent cerebrovascular complications are carotid-cavernous fistulas and arterial dissections. Extracranial and intracranial aneurysms have also been reported. The internal carotid artery (ICA) is the most common site of aneurysm formation, typically in the cavernous sinus. Rupture of various systemic and cerebral vessels leads to frequent bleeding and subarachnoid hemorrhage ( Fig. 3 ). Conventional angiography and angioplasty have a high rate of complications and therefore noninvasive techniques, such as MR angiography, are the primary investigation of choice in patients with EDS. Surgery is difficult in these patients because the friable arteries are difficult to suture.
Secondary Causes of Vessel Wall Abnormality
Many secondary or acquired conditions, such as infections, chemical or drugs, disorders of metabolism (vitamin C or substance P deficiency), atherosclerosis, or connective tissue diseases, may affect vessel wall leading to weakness in the vessel wall and predisposing them to bleed.
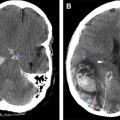
Septicemia, including meningococcemia and infective endocarditis ( Fig. 4 ), results in microbial damage to the brain microvasculature, effectively producing a type of vasculitis. This vessel wall damage can be caused by organisms directly infecting the vessel wall or damage to blood vessels as they traverse purulent exudate in cisterns and along the cerebritis bed. Secondary vessel damage can be caused by postinfectious inflammatory toxins, such as immunoglobulin, complement, lipoprotein, viral antigen or immune complex deposition, cold agglutinin formation, or vascular endothelial cell proliferation leading to vessel damage.
The proximal middle cerebral artery (MCA) and arteries in the posterior perforated substance are most often involved. MRI may show meningeal inflammatory changes as hyperintense signal in the basal cisterns and subarachnoid space on fluid attenuation inversion recovery images with thick enhancement on the postcontrast scans. Diffusion-weighted imaging may show changes of cerebritis, and associated acute brain infarction, whereas susceptibility-weighted imaging may show microhemorrhages from small vessel fragility. Hemorrhages in septicemia may also be caused by disseminated intravascular coagulation (DIC), discussed later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree