Protein Synthesis and Regulation
In eukaryotic cells, nuclear protein-encoding genes are transcribed into pre-mRNA molecules (also named heterogeneous-nuclear) that are transported to the cytoplasm after complex processing. In the cytoplasm, mRNAs become templates for translation, a highly energy-consuming process. Beyond 80S ribosomes and aminoacyl-tRNAs, several soluble factors and GTP are required for the initiation, elongation, and termination (also named release) steps of protein synthesis. Accordingly to the stage of translation in which they transiently associate with the ribosomes, the soluble factors are named eIFs (initiation factors), eEFs (elongation factors), and eRFs (releasing factors) with the prefix e- indicating eukaryote. Several ribosomes that are simultaneously engaged in the translation of the same mRNA form a polyribosome (or polysome). Polysomes are either free in the cytosol or bound to the surface of ER membranes.
Translation is also an important target of regulation. Several examples of regulatory mechanisms that control the expression of specific mRNAs in response to a given cue have been reported. The expression of some proteins involved in iron metabolism is regulated at the level of translation initiation or stability of mRNAs.
1,2 Besides specific mechanisms, global translational control exists. A transient arrest of translation is often required to overcome various kinds of stress. For instance, the sudden onset of an unfavorable condition, such as amino acid starvation, heat shock, accumulation of unfolded proteins in the ER, or lack of heme in reticulocytes, triggers a rapid halt in global protein synthesis. This regulation is mediated by specific sensor protein kinases that phosphorylate the alpha subunit of heterotrimeric initiation factor eIF2, a G-protein, on residue Ser51. eIF2a phosphorylation prevents the GDP-GTP exchange on eIF2 because the guanine nucleotide exchange factor (GEF) eIF2B is sequestered by tight binding to the phosphorylated form of eIF2. The GTP-bound form of eIF2 becomes insufficient for the formation of the ternary complex (eIF2-GTPMet-tRNA
i), and protein synthesis slows down. In contrast, ligands that activate signal transduction pathways promoting growth rapidly stimulate protein synthesis. Insulin signalling exemplifies a growth promoting pathway that, through the protein kinase mTOR, activates components of the translational machinery including eIFs and eEFs.
3,4Another site of protein synthesis is mitochondria that possess a genetic system and independent mRNA translation machinery. However, mitochondrial protein synthesis, which is from bacterial origin and occurs on 55S ribosomes present in the matrix, is responsible for the synthesis of only a small set of highly hydrophobic proteins that reside within the mitochondrion inner membrane. The rest of the mitochondrial proteins are nuclear encoded, translated on cytoplasmic ribosomes, imported from the cytoplasm, and sorted to the outer membrane, inner membrane, or matrix.
The broad spectrum of functions typical of a specialized cell of metazoans depends on a defined set of proteins that is referred to as the “proteome.” The proteome is specific for a
particular physiological cellular condition or stage of development. Furthermore, many proteins are modified by covalent attachment of chemical groups that contribute to increase the complexity of proteins produced by a cell. The analysis of the proteome and its modifications, which is carried out by a huge armamentarium of technologies that were developed in recent years, highlights the qualitative and quantitative changes occurring in pathological conditions with respect to the normal and greatly contributes to the identification of the molecular mechanisms fundamental to the basis of human disease.
Protein Sorting in the Cytoplasm
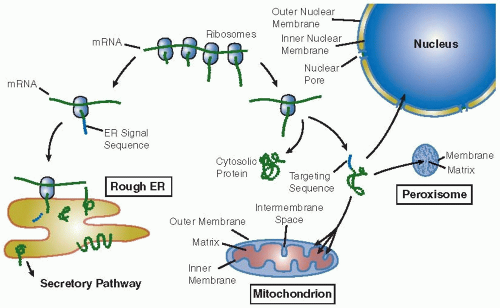
The great majority of cellular proteins are synthesized on “free” ribosomes in the cytosol and then transported to their eventual destination. Short linear sequences (or three-dimensional patches of particular amino acids) and their cognate receptors govern the sorting events. The sorting decision occurs after approximately 30 amino acids of the nascent polypeptide emerge from the ribosome. In the absence of an “ER signal sequence,” most often found near the amino-terminal end of the protein, translation is completed in the cytosol. The protein can either stay in the cytosol or be posttranslationally (after synthesis) transported into one of the organelles (
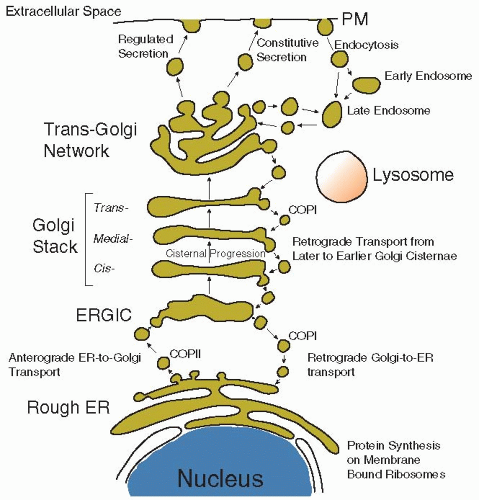
FIGURE 5.1). Alternatively, if the protein does contain an amino-terminal signal sequence, the nascent peptide-ribosome complex is docked on the membrane of the ER and the emerging polypeptide is extruded cotranslationally (during synthesis) into the lumen of this organelle. This marks the entry of the protein into the “secretory pathway,” a route that comprises the rough ER-Golgi-plasma membrane-lysosomes and through which a protein can reach the cell surface (
FIGURE 5.2). The sorting signals to the various organelles are listed in
Table 5.1.
Most of the proteins synthesized on “free” polysomes remain in the cytosol as cytosolic or “soluble” proteins. These include enzymes involved in glycolysis, signal transduction (e.g., soluble protein kinases or phosphatases), factors of the translation machinery, and components of the cytoskeleton. Different mechanisms operate to posttranslationally translocate the cytosolic proteins into the nucleus, mitochondrion, and peroxisome.
5,6,7The nucleus not only imports proteins but also exports proteins to the cytoplasm, and in this sense, the transport in this organelle is bidirectional and regulated. As shown in
Table 5.1, nuclear localization (NLS) and nuclear export (NES) signals were identified and shown to be highly selective for nuclear proteins.
8,9 Some proteins that shuttle from the nucleus to the cytoplasm contain both signals.
Protein Folding and Maturation
In order to become a functional protein, the nascent polypeptide chain must collapse into a conformation that is dictated primarily by the amino acid sequence (primary structure). Although this process can occur spontaneously at very low protein concentrations in a test tube, emerging polypeptides require assistance to fold in the highly crowded environment of the cytosol as the protein concentration is approximately 200 to 300 mg/mL. In fact, inappropriate interactions, misfolding, or aggregation occur under these conditions. Thus, nascent chains are guided through their maturation by molecular chaperones that also prevent inappropriate aggregation. The population of chaperones that assist folding in the cytosol is distinct from those that operate within the ER or mitochondria. Most molecular chaperones are members of the heat-shock protein (Hsp) family, so called because their expression is induced by briefly heating cells to 42°C. In general, chaperones attach to nascent polypeptide chains and initiate the folding process even before translation is completed. As the nascent polypeptide is being extruded from the ribosome, it is bound by chaperones that
recognize short sequence motifs containing hydrophobic amino acids. By undergoing cycles of binding and release (linked to ATP hydrolysis), these chaperones assist the nascent polypeptide to attain its native conformation, one aspect of which is burying hydrophobic sequence motifs in the protein interior so that they no longer contact the hydrophilic environment of the cytosol. Some properly folded protein monomers are assembled with other proteins to form multisubunit complexes. This also is facilitated by chaperones. Considerable effort is continuously devoted to the understanding of the mechanistic basis of protein folding and chaperone function.
Ubiquitin Addition Marks Proteins for Degradation
Proteins that are not properly folded are targeted for degradation. Such proteins are marked for selective destruction and are degraded to amino acids that are recycled in synthesis of new proteins. Degradation of selected molecules is achieved in two major phases. First, the molecules are tagged with a 76-residue polypeptide, termed
ubiquitin, which is covalently attached to the target, and sequential attachment of multiple ubiquitin molecules can form a long polyubiquitin chain. Second, the polyubiquitylated molecules are ferried to a cytosolic ATP-dependent protease complex, the 26S proteasome, for destruction. The degradation of some mutated proteins that cannot fold is essential for the cell to prevent accumulation of misfolded or aggregated proteins.
10 Indeed, there is increasing evidence that many human diseases are due to accumulation or aggregation of misfolded proteins.
In addition to carrying out the disposal of damaged and misfolded proteins, ubiquitylation is also responsible for regulating a wide array of cellular processes including cellular differentiation, tissue development, inflammatory responses, antigen presentation, cell cycle progression, and apoptosis.
11,12,13,14 This regulation is accomplished through the controlled destruction of selected key regulatory proteins.
Other forms of attachment of ubiquitin (mono- or polyubiquitylation) trigger other types of processes such as endocytosis and DNA repair. Recently, histones were found to be a target of monoubiquitylation that is implicated in the recruitment for nuclear processes such as transcription and DNA repair.
15
Targeting of Cytosolic Proteins to Membranes via Lipid Attachment
Some cytosolic proteins require the covalent attachment of lipid moieties in order to be inserted into a membrane where they will exert their function. The three main types of lipid attachment are
N-myristoylation, prenylation, and palmitoylation.
N-myristoylation consists of the attachment of a fatty acid of 14 carbon atoms (myristic acid) to the second residue, an invariable glycine, after proteolytic removal of the
N-terminal methionine. This process is predominantly cotranslational, mediated by soluble enzymes, and has a strict consensus sequence (M
GXXXS/T). The replacement of glycine with alanine abolishes the membrane attachment and consequently prevents the function of the protein at its site of action. This modification occurs in important regulatory proteins such as the proto-oncogene c-Src that is a protein kinase involved in the control of cell proliferation. More recently, members of the Ras family of small GTPases, which act as molecular GDP/GTP switches in many cellular processes, were found to be myristoylated. Among them are the ADP ribosylation factors (Arfs) that regulate membrane traffic through the secretory pathway and are ubiquitously expressed from yeast to humans
16 (see below). The best-characterized member of the Arf family is the yeast Arf1 protein. However, the mechanism of myristoylation of Arfs is not yet fully understood.
Prenylation is a posttranslational process also catalyzed by soluble enzymes. It involves the attachment of a prenyl group—either a farnesyl or a geranyl of 15 or 20 carbon atoms respectively—onto soluble proteins that carry a CaaX motif (C is cysteine, a is an aliphatic amino acid and × any amino acid) located at the C-terminal end of the protein. A prenyl group is attached to the cysteine residue, and then the last three amino acids are proteolytically removed and the cysteine is methylated. The Ras proteins undergo this modification that is otherwise rather rare. If the cysteine residue is mutated, the function of Ras in the cell is abolished because it cannot interact with its effectors. Since Ras proteins are activated in some kinds of cancer, inhibitors of prenylation are potential anticancer drugs.
As for the previous two modifications, also the attachment of a palmitic acid (a 16-C atoms fatty acid) to the SH group of a cysteine plays a role in the association of some proteins to the cytosolic side of the plasma membrane (S-palmitoylation). Polytopic membrane proteins catalyze this reaction, implying that it occurs at the cytosol-membrane interface. Palmitoylation is reversible and is often coupled with either
N-myristoylation or prenylation to regulate the strength of membrane interaction of soluble proteins. Palmitoylation is more than a simple sorting signal. It has been implicated in the process of protein trafficking between organelles, in the segregation or clustering of proteins in the membrane compartment, and its regulatory role is often associated with the reversibility of this modification.
17 It was also reported that covalent attachment of fatty acid to the amino acid in proximity of a transmembrane region provides an additional membrane anchor to the protein. One of such examples is the E2 glycoprotein of the Sindbis virus.
Posttranslational Modifications and Regulation of Protein Function
Many proteins are subjected to further processing in order to become active. Among the vast array of dynamic or persistent modifications of the amino acid backbone, the most common modifications essential for protein functionality are the phosphorylation of specific amino acids or the proteolytic cleavage of a region (propeptide) of the protein precursor.
Protein phosphorylation is a reversible covalent modification catalyzed by protein kinases that transfer the γ-phosphate from ATP to specific hydroxyl amino acids such as serine (95% of the phosphorylated sites), threonine (4%), or more rarely tyrosine (<1%). Protein kinases represent one of the largest families of proteins within the human genome counting about 700 members. The opposite reaction is catalyzed by protein phosphatases that hydrolyze the linkage between the phosphate and the amino acid. Protein phosphorylation influences protein conformation. Cycles of phosphorylation-dephosphorylation control the functionality of many soluble enzymes involved in key steps of metabolic pathways or of plasma membrane proteins such as membrane receptors that are usually phosphorylated in their cytosolic domain upon stimulation by an extracellular ligand. An additional role of phosphorylation of specific residues is to
create docking sites for the binding to other proteins. This occurs in many signal transduction pathways where extracellular signals trigger the phosphorylation of specific amino acids to create binding sites for adaptor proteins that in turn recruit other proteins and activate a downstream cascade of intracellular signalling that culminates in the activation of transcription of responsive genes.
Characterization of the “phosphoproteome” is important to identify new substrates of protein kinases or compare the profile of phosphorylated proteins in different physiological conditions.
18 This can open the possibility to study on a large scale the biology of the response to the activation or inhibition of specific cellular receptors.
18Proteolysis is a terminal modification that is often used to activate in a controlled and irreversible manner the precursors of proteins whose activity is potentially harmful. In general, proteases are synthesized as longer precursors (zymogens) that are cleaved to produce the active protein (see further proteolysis in the Golgi). Other mechanisms for regulating protein activity are the interaction with ions or small molecules, such as Ca2+, cyclic AMP, or guanylic nucleotides or with other protein partners.