Common gram-positive pathogens
Coagulase-negative staphylococci
Staphylococcus aureus, including methicillin-resistant strains
Enterococcus species, including vancomycin-resistant strains
Viridans group streptococci
Streptococcus pneumoniae
Streptococcus pyogenes
Common gram-negative pathogens
Escherichia coli
Klebsiella spp.
Enterobacter spp.
Pseudomonas aeruginosa
Citrobacter spp.
Acinetobacter spp.
Stenotrophomonas maltophilia
Initial management. Fever in patients with neutropenia should be considered as a manifestation of infection, and patients should promptly receive empirical antibiotic therapy, ideally within an hour of onset, as infection may progress rapidly. This may be difficult in many LMCIs, where access to care may delay initiation of antibiotics. The management of FN is influenced by patient characteristics, drug availability and cost, and local epidemiology. The management principles presented in this section are based on published guidelines [5, 7], but institution-specific guidelines need to be developed based on the antibiotic susceptibility pattern of organisms commonly isolated at a healthcare center.
The current tendency for the treatment of FN in many pediatric cancer centers (PCC) is the use of risk-stratification schemas. These schemas are based on factors related to patients (including age, type of malignancy, disease status), the type and timing of chemotherapy, and both clinical and laboratory characteristics of the specific FN episode, such as height of fever, hypotension, mucositis, blood counts, and C-reactive protein levels [7]. Patients at risk for invasive bacterial infection and clinical complications are those with anticipated prolonged and profound neutropenia (>7 days duration) following chemotherapy (ANC <100 cells/μL), and important comorbid conditions, including unstable vital signs, pneumonia, abdominal pain, and mental status changes [5, 7]. Validated stratification schemas in pediatrics exist, but none have been validated across various geographic locations. However, it is recommended that each institution use and keep a record of performance of a validated risk-stratification schema [7].
Laboratory and imaging studies. If the patient has no central venous catheter (CVC), ideally two sets of peripheral blood cultures should be obtained. If the patient has a CVC, blood specimens from each lumen should be obtained. Obtaining a peripheral blood culture in patients with CVCs is recommended. Chest radiographs are required only if there are pulmonary signs or symptoms.
Antibiotic treatment. Empiric antibiotic therapy for patients with FN needs to provide coverage for gram-negative organisms, including P. aeruginosa. Monotherapy regimens evaluated in children include antipseudomonal penicillins (piperacillin-tazobactam and ticarcillin-clavulanic acid), antipseudomonal cephalosporins (cefepime), and carbapenems (meropenem or imipenem). Antipseudomonal penicillins are an excellent option for initial monotherapy if cefepime is not available. Carbapenems should be conserved for broadening antibiotic spectrum and for intrabdominal process (see section on typhlitis). Ceftazidime or aminoglycosides are not sufficient as monotherapy because of intrinsic and acquired resistance among gram-negative organisms [8]. Aminoglycosides and/or fluoroquinolones may be added to the initial regimen to manage complications (evidence of sepsis or pneumonia) or if antimicrobial resistance is suspected [5]. Vancomycin is not recommended as part of the initial antibiotic regimen for FN, unless there are specific clinical indications (Table 7.2) or the center reports a high rate of resistant pathogens [5].
Table 7.2
Indications for addition of antibiotics active against gram-positive organisms to the empirical regimen for fever and neutropenia
Hemodynamic instability or other evidence of severe sepsis |
Pneumonia documented radiographically |
Positive blood culture for gram-positive bacteria, before final identification and susceptibility testing is available |
Clinically suspected serious catheter-related infection (e.g., chills or rigors with infusion through catheter and cellulitis around the catheter entry/exit site) |
Skin or soft-tissue infection at any site |
Colonization with methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, or penicillin-resistant Streptococcus pneumoniae |
Severe mucositis, if fluoroquinolone prophylaxis has been given and ceftazidime is employed as empirical therapy |
Empiric antifungal treatment can be initiated in children at high risk for IFD who have persistent fever despite prolonged (>96 h) broad-spectrum antibiotic therapy. Currently, caspofungin or liposomal amphotericin B (L-AmB) is recommended for empiric antifungal therapy [9]. If these compounds are not available, amphotericin B (AmB) deoxycholate is equally effective. Close monitoring of renal and hepatic function is recommended to avoid toxicity.
Ongoing management. Initial antibacterials should be modified only if clinical, microbiological, or imaging changes indicate a new infection. In patients who respond to initial empiric antibiotic therapy, double coverage for gram-negative infection and glycopeptides can discontinued after 24–72 h if susceptible bacteria are not recovered in cultures. In children with persistent fever who become clinically unstable, the initial empiric antibacterial regimen can be escalated to include coverage for resistant gram-negative, gram-positive, and anaerobic bacteria, particularly if blood culture results are indicative of resistant bacteria [7].
Duration of antibiotic treatment. Empiric antibiotics can be discontinued in patients with negative blood cultures at 48 h, who have been afebrile for at least 24 h, and who have evidence of marrow recovery (increasing ANC >500 cells/μL). Empiric antibiotics may be discontinued at 72 h for low-risk patients who have negative blood cultures and have been afebrile for at least 24 h, irrespective of marrow recovery status, only if close follow up is possible [7].
In patients with clinically or microbiologically documented infections, the duration of therapy is dictated by the particular organism and site. Appropriate antibiotics should be continued for at least the duration of neutropenia (until ANC is >500 cells/μL) or longer if clinically necessary. Alternatively, if an appropriate treatment course has been completed and all signs and symptoms of a documented infection have resolved, oral fluoroquinolone can be administered until marrow recovery in patients who remain neutropenic [5] (Table 7.3).
Table 7.3
Suggested empiric antibiotic therapy for patients with febrile neutropenia
Suggested first line therapy needs to provide coverage for gram-negative organisms including P. aeruginosa | 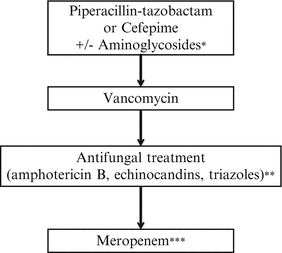 |
Fever persists after 48-h of treatment/suspected Staphylococcal infection | |
Persistent fever beyond 96-h, add empiric antifungal treatment | |
No improvement/suspected hospital-acquired infection/neutropenic colitis |
Central Venous Catheter Infections
Classification. CVC infections are classified according to the primary site of infection. An exit-site infection is a superficial infection at the site of insertion with erythema, tenderness, induration, or purulence within 2 cm of the exit of the CVC or edge of the subcutaneous port (SP). Tunnel tract infections affect the subcutaneous tissue surrounding the CVC or SP at a distance greater than 2 cm from the skin exit. An infection of the subcutaneous tissue surrounding a SP is referred to as a pocket infection. A catheter-related bloodstream infection (CRBI) is a laboratory-confirmed bacteremia or fungemia arising from microbial colonization of a CVC or SP, which may be associated with systemic symptoms. Septic thrombosis can occur as a complication of CVC infections [10].
Etiology. The most frequently isolated organisms are Gram-positive bacteria, the most common being coagulase-negative Staphylococcus. Other organisms include S. aureus, Streptococcus viridans, enterococcus, gram-negative organisms (E. coli, Klebsiella spp., or Enterobacter spp.), and Pseudomonas aeruginosa. Bacillus species, Mycobacterium chelonae, and Mycobacterium fortuitum are less frequent. Candida spp. are the most commonly isolated fungal organism [10].
Diagnosis. For exit-site infections, culture of discharge or wound aspirate is recommended. A swab specimen from the exit site is acceptable, but may be contaminated with skin flora. Gram staining, staining for fungal organisms and acid-fast bacilli (AFB), and routine bacterial and fungal cultures should be performed. AFB culture should be ordered if there is no improvement after 48–72 h of therapy. Diagnosis of CRBI is best made by collecting paired CVC and peripheral vein (PV) blood cultures. All lumens of all indwelling intravenous catheters should be cultured simultaneously. A diagnosis of CRBI can be made by comparing the time to detection (TD) [11] of paired CVC and PV cultures. If the TD of blood cultures drawn through the suspected CVC is at least 120 min less than the TD of PV drawn at the same time, a CRBI is likely [11]. If only paired cultures from two lumens of a CVC are available, a CRBI is likely if TD of Lumen 1 is 120 min or more of the TD of Lumen 2 [12].
Indications for removal of catheters. The CVC or SP should be removed if no longer needed. Catheters should be also removed if (1) the patient’s clinical condition deteriorates, (2) there are signs of sepsis, (3) blood cultures remain positive after 48–72 h of effective therapy, and (4) if CRBI relapses. Infections by S. aureus, Candida spp., and Malassezia spp., also require catheter removal. Removal should be strongly considered in bacteremia caused by Bacillus spp., rapidly growing Mycobacterium spp., and Gram-negative bacilli, as infection may be very difficult to eradicate without line removal [10]. Catheters should also be removed if septic thrombosis is present.
Management. Most exit-site infections can be managed with local care and topical antibiotics in non-neutropenic patients. Patients with mild to moderate signs and symptoms, but without fever, may be treated with oral antibiotics such as dicloxacillin, amoxicillin/clavulanic acid, or oral cephalosporin. Clindamycin may be used if MRSA is suspected. All patients with fever or rapidly progressing infections should be treated with parenteral antibiotics. Initial antibiotic therapy should include a broad-spectrum antipseudomonal penicillin or cephalosporin plus vancomycin. If infection with gram-negative bacteria is suspected, an aminoglycoside should be added. If the patient has severe abdominal pain or clinical symptoms of sepsis, the antipseudomonal β-lactams can be substituted with carbapenems. Definitive antibiotic therapy should be based on susceptibility testing of isolated pathogens.
Exit-site infections that improve promptly should be treated for 5–7 days. Infections of the tunnel tract or pocket of the SP usually require removal of the CVC or SP and at least 7–10 days of appropriate antimicrobial therapy. Patients with CRBI who respond promptly to therapy and have no complications should receive 10–14 days of antibiotic therapy. For infections due to yeasts, antifungals should be given for 14 days after the last positive blood culture after removal of catheter. In local peripheral vein thrombosis with purulent collection, surgical debridement might be needed. For a central blood vessel thrombosis, an anticoagulant is recommended in addition to antibiotics. For septic embolism, antibiotic therapy may be continued for 4–6 weeks. The CVC or SP should not be reinserted until effective parenteral antibiotic therapy is begun and repeat blood cultures yield negative results [13].
Antibiotic dwell therapy consists in instilling the lumen of the infected catheter with a high concentration (1–5 mg/mL) of an antibiotic surpassing the minimal inhibitory concentration to which the isolated microorganism is susceptible and closing the lumen for 8–12 h. In patients with a single lumen catheter for which sufficient dwell time cannot be arranged, a peripheral IV to administer other medications should be considered. Dwell therapy is recommended for as much of the 10- to 14-day treatment course as possible [13].
Respiratory Infections
Cancer patients are at increased risk for respiratory infections. The diagnosis of pulmonary infections in immunocompromised patients can be challenging because of atypical presentations and the absence of localizing signs and symptoms, particularly during severe neutropenia. Most patients present with nonspecific symptoms such as cough and fever, which may be accompanied by tachypnea and dyspnea in more severe cases. Local epidemiology, the setting from which the patient presents (community vs. hospital), and seasonality of viral infections may also aid in the etiological diagnosis.
Diagnosis. Nasopharyngeal washings or swabs are useful to diagnose viral infection. Polymerase chain reaction methods are currently the gold standard for diagnosis of respiratory viruses. If not available, samples may be sent for direct fluorescent antibody analysis and viral culture [14]. Serologic studies may be performed to identify organisms producing atypical pneumonia and other organisms (cytomegalovirus, Cryptococcus, Aspergillus). If tuberculosis is suspected, sputum or gastric aspirates for acid fast stain and culture should be obtained. A tuberculin skin test (TST) may aid in the diagnosis; however, a negative test does not rule out tuberculosis. Interferon-gamma release assays (IGRAs) indicate the immune response to Mycobacterium tuberculosis but does not differentiate between active and latent infections. Similarly to TST, a negative result of IGRA may not rule out active tuberculosis. Access to this type of study is still limited in most LMICs. Urinary antigen tests can be used to diagnose Legionella pneumophila and Histoplasma capsulatum. Blood cultures should be obtained from all immunosuppressed patients with pneumonia [15].
Etiological diagnosis of respiratory infections may be a challenge in most LMCIs, particularly if microbiological tests are not available. In this scenario, imaging studies may provide additional clues to the etiology of infection. A radiologic pattern of lobar or segmental pneumonia with reactive pleural effusion is indicative of bacterial organisms. In hospital-acquired pneumonia, infiltrates can be bilateral with diffuse or multilobar consolidation and pleural effusions. Atypical pneumonia may present with unilateral or bilateral infiltrates with a segmental distribution or as a diffuse bilateral reticular nodular infiltrate. In viral pneumonia, a radiologic pattern of poorly defined nodules and patchy areas of peribronchial ground glass opacity is seen. A diffuse pattern of pulmonary infiltrates may also suggest infection with Legionella spp., fungi, or parasites. The presence of nodules, with or without cavitations, suggests infection by molds or mycobacteria. Miliary lesions suggest tuberculosis, histoplasmosis, or coccidiomycosis. Radiologic findings of pneumocystis pneumonia reveal diffuse, bilateral, interstitial, or alveolar infiltrates [15, 16]. In patients with neutropenia, radiographic evidence of pneumonia may appear only with immune reconstitution. Computed tomography, if available, is more sensitive and specific than chest radiography in these patients [16].
Bronchoscopy is suggested if persistent infiltrate, nodules, or masses are present and to obtain specimens for microbiological analysis. Patients with an endotracheal tube in place may require flexible bronchoscopy for bronchoalveolar lavage for cultures. Lung biopsy is used to obtain a definitive diagnosis of pneumonia in immunosuppressed patients with a pulmonary infiltrate. A previous nondiagnostic bronchoscopy or the need for larger specimens of tissue for diagnosis may support the need for thoracoscopic or open lung biopsy [15].
Management. Empiric therapy may be guided by local epidemiology and imaging. If the radiographic image shows focal lesions suggestive of bacterial infection, broad-spectrum antibiotics against gram-positive and gram-negative organisms should be given and the response evaluated after 48–72 h. If there is good response to antibiotics, treatment can be continued for at least 2 weeks if etiological diagnosis is not available. If broad-spectrum antibiotics are not effective, empiric treatment for organisms such as Pneumocystis carinii (using TMP-SMX), Legionella spp., or Mycoplasma spp. (using macrolides), and viruses (i.e., influenza virus) should be initiated. Frequently, susceptibility testing for influenza is not available in LMICs. Empiric treatment using oseltamivir may be initiated. Tuberculosis should be treated with a four-drug regimen, determined by local epidemiology. Empiric antifungal treatment can be given to patients with pulmonary nodules or cavitation or who develop new pulmonary infiltrates while receiving broad-spectrum antibiotics. Pulmonary fungal infections, particularly molds, may require longer therapy (up to months), which should be continued through periods of immunosuppression, until resolution of clinical findings and improvement of lesions in imaging studies [15].
Fungal Infections
Patients with acute myeloid leukemia or relapsed acute leukemia, patients receiving highly myelosuppressive chemotherapy for other malignancies, and allogeneic HSCT recipients who are expected to present with prolonged neutropenia (>10 days) are at high risk for IFD [7].
Candida species can cause bloodstream infection and disseminated disease such as hepatosplenic candidiasis. Mold infections, including aspergillosis, zygomycosis, and fusariosis, occur in patients with profound (<100 cells/μL) and prolonged (>10 days) neutropenia, and may affect lungs, sinuses, and soft tissues [16]. Early diagnosis is difficult due to nonspecific signs and symptoms; in early stages, persistent or recurrent fever may be the only sign of infection. In advanced infection, organ system involvement may be evident. Blood cultures are useful for the diagnosis of yeast infections, but not usually for mold infections. Galactomannan antigens have been validated as a surrogate marker for detection of invasive aspergillosis in patients with hematological malignancy [16]. Tissue obtained from lesions should be sent for fungal stains, culture, and histopathology.
Radiographic imaging such as CT may reveal abnormalities in either the lungs or sinuses. Pulmonary macronodules with or without a halo sign are the most typical findings associated with invasive aspergillosis and are evident during neutropenia. Other manifestations include nodular, wedge-shaped, peripheral, multiple, or cavitary lesions. In hepatosplenic candidemia, ultrasonography will reveal characteristic “bull’s-eye” or “target” lesion with a hypoechoic mass containing a hyperechoic center. CT images may reveal areas of low density in the liver, spleen, and, sometimes, the kidney. A radiologic abnormality is more obvious after the resolution of neutropenia [16].
Empirical antifungal therapy is indicated in patients with persistent or recrudescent FN. The choice of empirical antifungal agent depends upon the likely fungal pathogens, toxicities, and cost. Prophylaxis with fluconazole reduces the incidence of invasive Candida infections but has no activity against molds. In patients who have not received antifungal prophylaxis, candidemia is initially the greatest concern. For patients receiving prophylaxis, infections by fluconazole-resistant strains or molds are more likely [9]. AmB desoxycholate has been the standard empirical choice. Empiric therapy with other antifungal agents, including alternate formulations of AmB, azoles with mold activity (itraconazole or voriconazole), and echinocandins (caspofungin) have been described. None have proven to have greater efficacy, but are generally less toxic than AmB [16]. For patients already receiving mold-active prophylaxis, switching to an IV anti-mold agent within a different antifungal class is recommended. Therapy should be continued until lesions have resolved (usually months) and through periods of immunosuppression (e.g., chemotherapy and transplantation) [9]. If culture of lesions is obtained, susceptibility testing should guide treatment.
Gastrointestinal Infections
Chemotherapy leads to mucositis, characterized by atrophy of the gastrointestinal (GI) mucosa, degeneration of the connective tissue, and ulceration. It is frequent among patients receiving high-dose methotrexate, cyclophosphamide and cytarabine, or anthracyclines.
Oral mucositis. Oral mucositis can be a port of entry for infectious agents, which may predispose the patient to local and severe systemic infections. Infections generally reflect the oral microbiome, such as viridans group of streptococci. Reactivation of herpetic lesions is common. Other contributing factors are periodontal disease, inadequate oral hygiene, xerostomia, and poor nutritional status. The severity of oral mucositis is assessed by the Common Toxicity Criteria for Oral Mucositis by the National Cancer Institute (NCI) and the Oral Toxicity Score of the World Health Organization (WHO) (Table 7.4).
Table 7.4
Scales for assessment of chemotherapy-induced mucositis
World Health Organization Mucositis Toxicity Scale |
0 = None |
1 = Soreness ± erythema |
2 = Erythema, ulcers, patient can swallow food |
3 = Ulcers with extensive erythema, patient cannot swallow solid food |
4 = Alimentation is not possible |
National Cancer Institute Common Toxicity Criteria for Oral Mucositis |
Appearance score |
0 = None |
1 = Erythema of the mucosa |
2 = Patchy ulcerations or pseudomembranes |
3 = Confluent ulcerations or pseudomembranes, bleeding with minor trauma |
4 = Tissue necrosis, significant spontaneous bleeding, life-threatening consequences |
Functional/symptomatic score |
1 = Ability to eat solids |
2 = Requires liquid diet |
3 = Not able to tolerate a solid or liquid diet |
4 = Symptoms associated with life-threatening consequences |
The management of mild, uncomplicated oral mucositis is through oral hygiene and antibacterial mouthwashes. No antibiotic therapy is recommended. For infection associated with fever, localized ulceration, or lymphadenitis in patients with neutropenia, broad-spectrum antibiotic therapy is recommended. In patients with grade 3 or greater mucositis, vancomycin should be added for coverage against viridans streptococci and intravenous clindamycin for empiric coverage against Capnocytophaga spp. [17]. In patients who have tender cervical lymphadenopathy while receiving broad-spectrum antibiotics, antifungal agents should be considered for lymphadenitis due to Candida spp. [18]. For herpetic lesions, acyclovir should be given.
Enteric infections. Enteric infections are common public health issues in many LMCIs. Infections in children with cancer can be produced by community-acquired pathogens, including bacteria, viruses, and parasites. Other microorganisms (Aeromonas spp., Listeria monocytogenes, Salmonella spp., Clostridium difficile, cytomegalovirus, adenovirus, Candida spp., Strongyloides stercoralis, and Cryptosporidium spp.) are also known to cause enteric infection in immunocompromised hosts [19]. Most enteric pathogens are transmitted by the oral–fecal route. Risk factors include contaminated food or water, and contact with animals. Prolonged hospitalization and exposure to other infected patients can put cancer patients at risk for hospital-acquired enteritis. In patients receiving broad-spectrum antibiotics, Clostridium difficile may cause watery diarrhea, pseudomembranous colitis, or toxic megacolon.
For the diagnosis, stool samples should be sent for white blood cell smear, routine microscopy (ova and parasites) modified acid fast stains (coccidian protozoa), antigen testing (rotavirus), C. difficile toxins, and cultures for enteric bacteria. If the patient with enteric symptoms has fever, blood cultures should be obtained.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree