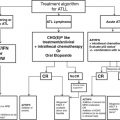
Emetic risk group
Risk (% pts.)
Acute prevention
Delayed prevention
High
>90 %
5-HT3 RA
DEX + aprepitant
+ DEX + (fos)aprepitant
AC combinations
–
5-HT3 RAa
Aprepitant
+ DEX + (fos)aprepitant
Moderate
30–90 %
Palonosetron + DEX
DEX
Low
10–30 %
Single agent (DEX, 5-HT3 DRA)
No routine prophylaxis
Minimal
<10 %
No routine prophylaxis
No routine prophylaxis
The above recommendations can often not be applied. As indicated by the BHGI group [3], at basic levels of resource allocation, hydration therapy should be available (oral and IV hydration and electrolyte replacement). Available antiemetics should include corticosteroids, prokinetic, nonselective serotonin antagonists, and dopamine receptor antagonists. At limited levels, antiemetics should include selective serotonin (5-HT3) antagonists. H2 antagonists could be considered to counteract steroid side effects. At enhanced levels, newer effective antiemetics, such as NK-1 antagonists, may be used with highly emetogenic chemotherapy. Proton pump inhibitors (PPIs) may be considered to counteract steroid side effects.
3 Febrile Neutropenia
Prevention of febrile neutropenia is essential as this complication can lead to death, especially in patients with comorbidities. Furthermore, in curative settings, maintenance of adequate dose intensity of chemotherapy leads to better chances of patient survival. Febrile neutropenia (FN) is defined as an oral temperature >38.5 C or two consecutive readings of >38.0 C for 2 h and an absolute neutrophil count <0.5 * 109/l, or expected to fall below 0.5 * 109/l [3]. Evidence-based guidelines identify a risk of more than 20 % of febrile neutropenia as a reason to use primary prophylaxis with G-CSF and indicate that in cases where a predicted FN risk varies around 10–20 %, other risk factors have to be considered, with age more than 65 clearly increasing the risk of FN [6].
There are various forms of G-CSF available: originators and biosimilars, from short acting like filgrastim or lenograstim to longer acting (needing only one injection) like pegfilgrastim and lipegfilgrastim. One should pay attention to the quality of these agents, and guidelines suggest to use those recognized by EMA or FDA and according to the label, i.e., beyond the day of nadir of white blood cell counts in case of short-acting agents. The European Organisation for Research and Treatment of Cancer (EORTC) guidelines, and others, indicate that the use of antibiotic prophylaxis (fluoroquinolones) to prevent infections in cancer patients at risk of neutropenia is generally not recommended and carries the risk of development of bacterial resistance. Of note, the UK National Institute for Health and Clinical Excellence has issued a position which considers this as an alternative to G-CSF usage [7]. There is no evidence specifically for or against this practice, but the development of antibiotic resistance, side effects, and drug interactions may be of concern.
It is suggested to identify patients at risk for complications if febrile neutropenia appears using risk indices such as those developed by the Multinational Association for Supportive Care in Cancer (See Table 2). This MASCC score, which has not been validated in a tropical environment, indicates that patients with a score of 21 or more points are considered at low risk and might be treated as outpatients in some conditions, while all other patients should be admitted to a clinical facility [8]. The type of antibiotic to be initially used is obviously related to the patient’s infectious disease history and prior antibiotic usage, and one has to take into account the particular strains of possibly resistant bacteria that the patient may have been exposed to. In stable patients, combinations (quinolone with amoxicillin plus clavulanic acid) are preferred over single-agent quinolones because most centers have seen a rise in Gram-positive FN episodes [9]. In high-risk cases, like those who will have prolonged neutropenia and those with proven bacteremia, intravenous combinations of a beta-lactam antibiotic with an aminoglycoside are preferable. In other high-risk patients, an anti-pseudomonal cephalosporin like ceftazidime or a carbapenem can be used initially.
Table 2
MASCC (Multinational Association for Supportive Care in Cancer) risk index
Score | Characteristic |
|---|---|
Burden of illness | |
5 | No or mild symptoms |
3 | Moderate symptoms |
5 | No hypotension |
4 | No chronic obstructive pulmonary disease |
4 | Solid tumor/lymphoma or no previous fungal infection |
3 | No dehydration |
3 | Outpatient status at onset of fever |
2 | Age <60 years |
However, many countries have had to restrict access to G-CSFs, even though their cost is decreasing with the advent of biosimilars. These are the BHGI recommendations: At basic levels of resource allocation, broad-spectrum antibiotics should be available. At limited levels, antifungal agents should be available. Red blood cell transfusion may be needed in case of acute anemia or bleeding, and consultation with a specialist regarding febrile neutropenia should be considered. At enhanced levels, additional therapies include and granulocyte growth factors.
4 Bone Health
Bone loss (osteoporosis) and associated fractures increase with patient age, for both sexes, and are also related to malnutrition, in particular lack of vitamin D which is frequent also among populations in sunny countries [10].
The World Health Organization indicates the following risk factors (other than low bone mineral density) for bone loss: age, gender (female), smoking, personal history of fracture after 50+ years, parental history of hip fracture, low body mass index (<20 kg/m2), consumption of more than three units of alcohol per day, corticosteroid use, and having other diseases (such as rheumatoid arthritis or vitamin D deficiency [11].
Aromatase inhibitors (AIs), both steroidal and nonsteroidal, provoke significant bone mineral density (BMD) decreases and they increase the fracture rate. Androgen deprivation therapy (ADT) is an important treatment approach in most men with prostate cancer. Definitive ADT can be achieved by orchiectomy, but is more commonly accomplished through the use of a gonadotropin-releasing hormone agonist (GnRH). This results in a reduction of both serum testosterone and estradiol, which in turn accelerates bone loss and significantly increases risk of fracture [12]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree