Chapter 68 Cardiovascular Disease in HIV
Cardiovascular disease (CVD) has emerged as a looming problem for patients infected with HIV, because of two principal factors. First, combination antiretroviral therapy (ART) has led to a dramatic improvement in survival prognosis rendering patients susceptible to age-related morbidities including CVD.1–3 Second, exposure to ART is associated with an accelerated risk of CVD4–7 likely by accelerating the progression of atherosclerosis. In addition to atherosclerosis and its varied clinical presentation, this chapter also outlines other cardiac problems seen in HIV-infected persons.
ATHEROSCLEROSIS IN HIV
The Time Course of the Pathology
Atherosclerosis is a condition within the arterial wall.8,9 The pathological evolution starts with the formation of fatty streaks, followed by lipid-rich plaque formation. LDL cholesterol exits the plasma and is engulfed in macrophages within the fatty streaks and plaques and inflammation develops.10 Over time, a lipid core possibly mixed with crystallized calcium is formed within a fibrous cap.
In the normal population, fatty streaks can be observed in 30–50% of otherwise healthy persons in their second and third decades of life11 and increase in number and extent thereafter. Fatty streaks and plaques are usually not associated with symptoms, but can become clinically detectable in either of two ways:
Risk Factors Other Than HIV and ART
A multitude of factors influence the risk of atherosclerosis and its clinical manifestations in HIV (in Table 68-1 suspected and documented risk factors in HIV-infected populations are outlined). Most of these risk factors are well described in the general population (except of course those related to HIV and ART), and the contribution to risk of IHD is fairly comparable in HIV-infected populations and in the general population.
Table 68-1 Risk Factors Known or Suspected to Influence the Risk of Ischemic Heart Disease (IHD) in HIV-Infected Persons
| Factor | Aggravates Traditional Risk Factors for IHD | Independent Predictor of IHD Risk in HIV? |
|---|---|---|
| Exposure to: | ||
| Antiretroviral treatment | Yes | Yes |
| Protease inhibitors | Yes | Yes |
| NNRTIs | No | No |
| NRTIs | Yes (stavudine) | ? |
| Age | – | Yes |
| Gender | – | Yes |
| Diabetes | – | Yes |
| History of CVD | – | Yes |
| Hypertension | – | Yes |
| Smoking | – | Yes |
| Family history of premature CVD | – | No |
| Total cholesterol | – | Yes |
| HDL cholesterol | – | Yes |
| Triglycerides | – | No |
| Replication of HIV | Yes | No |
| Level of immunodeficency | Yes | No |
| Development of lipoatrophy | Yes | No |
CVD: cardiovascular disease; IHD: ischemic heart disease.
Source: The Data collection of Adverse effects of anti-HIV drugs (D:A:D) study7 and updates from this study in 200545 and 2006.43
The current understanding of the risk factors depicted in Table 68.1 and their role in determining CVD/IHD risk in the general population and in HIV-infected populations is outlined below. Most of the knowledge cited below is derived from studies of the general population, and it appears reasonable to generally extrapolate most of this knowledge to the HIV-infected population. However, there are caveats surrounding certain aspects of doing such an extrapolation as noted below. Furthermore, the risk factors described in the table depict our current understanding of the situation in HIV, and this may be revised (expanded or reduced) as additional experience accumulates. Regardless, the success of preventive interventions is closely linked with intimate knowledge of this area.
Age and gender have already been mentioned.12 Of key relevance, the atherosclerotic process takes decades to develop, and lack of clinical symptoms should not necessarily lead to complacency in managing the cardiovascular health of a person. Conversely, risk factors for normal health other than CVD may very well take priority in younger patients infected with HIV, and the expected benefit versus the potential risk from interventions to prevent CVD should be carefully considered in younger patients also.
A family history of premature CVD also adversely affects the risk of CVD.13 The definition of ‘prematurity’ differs in various studies, but one operational definition is ‘CVD before the age of 50 in a first-degree relative’. In part, this risk factor is related to dietary and genetic predisposition of well-known risk factors (e.g., congenital lipid disorders), and hence the clinical relevance of this risk factor is low in populations extensively screened for the risk factors described below.
Patients with a history of CVD have a six- to ninefold increased risk of having a reoccurrence of a CVD compared with patients without such a history.14 Patients with diabetes mellitus have comparable excess risk.14,15 Hence, diabetes can be considered a so-called ‘CVD equivalent’. The reason why patients with diabetes have such a high risk of CVD is likely not linked to the abnormal glucose metabolism per se, whereas intense management of traditional CVD risk factors reduces the risk of CVD in patients with diabetes.16
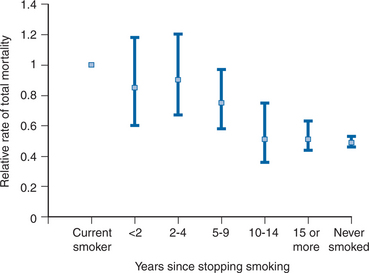
Smoking, especially cigarettes, accelerates the risk of CVD.17,18 Higher absolute consumption, longer duration of consumption, inhalation of the fumes, female gender, and younger age of the patient are all associated with relatively larger detrimental effects from smoking. Although the relative excess risk of CVD for smokers (vs nonsmokers) is greater for persons below 65 years of age, continued smoking also after this age is associated with excess risk.19 Conversely, cessation of smoking is associated with a gradual reduction in risk of CVD over the first 5 years (∼50% reduction in risk), whereas the detrimental influence on total mortality takes approximately twice as long to overcome (Fig. 68-1).20
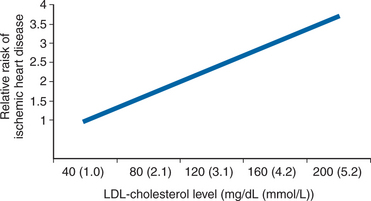
LDL cholesterol is an integral part of the pathology of atherosclerosis and it is therefore not surprising that the risk of CVD is closely positively correlated with the plasma levels of this lipid. Importantly, there is a linear correlation between LDL cholesterol levels and the risk of CVD without any lower threshold (Fig. 68-2).21 The level of LDL cholesterol is linked to diet with persons consuming a Western-type diet having the highest levels. Interventions aimed at reducing the plasma levels of LDL cholesterol have conclusively demonstrated a reduction in the risk of CVD.22
Conversely, HDL cholesterol tends to transport LDL cholesterol away from the arterial plaques, reduce it in size and hence slow the progressive atherosclerotic process. The level of HDL cholesterol is inversely correlated with the risk of CVD.22,23 Nonmedical interventions aimed at increasing HDL levels are associated with a reduced risk of CVD, whereas medical interventions principally designed to increase HDL cholesterol levels remain to be developed for routine clinical usage.
It is controversial whether fasting triglyceride plasma levels per se influence the risk of CVD. Elevated triglyceride levels are usually seen in conjunction with other CVD risk factors such as elevated LDL cholesterol, insulin resistance, and other manifestations of the metabolic syndrome (see discussion below), and it appears that once these other risk factors are controlled, the adverse association of high triglyceride and increased risk of IHD is removed.24,25 The combination of high triglycerides and low HDL cholesterol may, however, constitute a situation where the risk of IHD remains increased.26
Hypertension is a risk factor in many types of CVD, especially in stroke (see below).
Several versions of the definition of the metabolic syndrome exist.27 The principal components are, however, the same, namely; insulin resistance, abdominal obesity, dyslipidemia (low HDL cholesterol and high triglyceride levels), and hypertension.28 Much focus has been placed on this syndrome for two reasons. First, these factors are interlinked (perhaps causally) and coexist in many people, and second, it may be that patients with the metabolic syndrome have a risk of CVD that exceeds the contribution of the risk factors when present on their own. Whereas the first reason is definitively true, it remains to be clearly demonstrated whether the second reason is also true, especially in situations where drugs used in a population (e.g., ART, see below) are able to induce components of the syndrome. In the background population, the prevalence of the metabolic syndrome has increased markedly in recent times, predominantly because of an increasing prevalence of obesity. More studies are required in HIV-infected populations before the clinical relevance of assessing the metabolic syndrome in this population can be determined. However, as patients fulfilling the definition of the metabolic syndrome have several risk factors for CVD (as per definition), such patients should be targeted for interventions to modify each of these risk factors.
Physical inactivity is causally related with excess risk of CVD. There is a dose–response relation between baseline physical activity and health benefit. When sedentary, even a modest increase in physical activity is beneficial.29 The mechanisms by which physical activity decreases the risk of CVD include improvement in HDL cholesterol level,30 reduction in insulin resistance,31 lowering of body weight,32 and reduced blood pressure.33 Among patients with a history of IHD, exercise training reduces the need for revasculation procedures.34
Elevated C-reactive protein (CRP), determined by ultrasensitive (us) techniques, is associated with excess risk of CVD.35,36 However, the place for usCRP measurements in routine practice as a clinically useful determinant for assessing the risk of CVD remains to be defined, especially in HIV-infected populations where unrelated ongoing infection may increase usCRP levels as part of an acute inflammatory reaction.
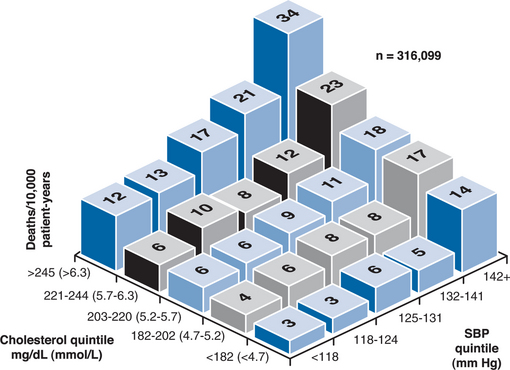
Of key importance, the factors described above that contribute to the risk of CVD do so independently of each other. That is, when more than one factor is present in an individual patient, the CVD risk factors increase additively (Fig. 68-3).37
HIV Infection as a CVD Risk Factor
Advanced untreated and treated HIV infection is associated with reduced total, LDL-, and HDL-cholesterol levels and hypertriglyceridemia.38–40 The risk of IHD in untreated HIV infection is low in absolute terms7 and lower than what would be expected when assessing the expected IHD risk from risk equations used to assess risk of IHD in the background population.41 It may be hypothesized that the metabolic alterations and the immunodeficiency may adversely affect the risk of CVD in untreated HIV infection, but the information available so far does not suggest that this is of major clinical importance.
ART as a CVD Risk Factor
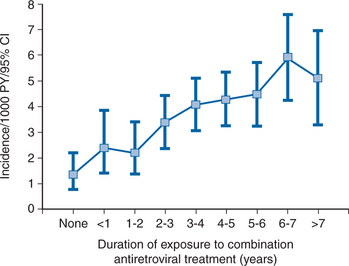
Several studies have consistently shown that duration of exposure to ART is associated with a gradually increased risk of IHD in particular4,6,7 (Fig. 68-4) and CVD in general.5 This adverse effect of ART is at least partly driven via the effects that components of ART have on various metabolic factors, most prominently LDL-cholesterol elevation (see Chapters 71 and 72). In populations exposed to antiretrovirals, elevation of cholesterol is associated with suppressed viral replication and higher CD4+ T-lymphocyte counts42 suggesting that ART reverses (and possibly exacerbates) the reductions in total cholesterol seen in advanced cases of untreated HIV.
Not all drug classes and not all drugs within a class are likely associated with comparable risk. Presently, it appears that an excess risk is carried from exposure to drugs within the protease inhibitor class.43 Some (but not all)44 of this effect is mediated by how these drugs affect cholesterol metabolism, in particular LDL levels. Whether all individual drugs within this class affect the risk of IHD equally remains to be determined; some differences for example exist in how these drugs affect lipids (see Chapter 72).
The size of the added risk for IHD from being exposed to a drug from the protease inhibitor drug class has been expressed in the literature as the added risk per year of exposure.43,45 This added risk amounts to 16% per year of exposure.43 However, as this risk accumulates for each additional year of exposure, for example, 5 years of exposure is equal to 110% (1.165 = 2.10 (i.e., slightly more than a doubling of risk)). However, as mentioned, some of this added risk is mediated via how these drugs affect lipid levels. Once lipids are controlled for, the remaining added risk per year of exposure to the protease inhibitor class is 10% (60% per 5 years of exposure).43 Additionally, these projections are based on the assumption that the added risk per year of exposure is comparable for each year of exposure. This assumption appears valid for up to 7 years of exposure (Fig. 68-4), whereas it remains unknown whether it is also true for even longer periods of exposure. Hence, extrapolations of these risk estimates for decades of exposure should not be done until additional experience has accumulated.
Conversely, exposure to the two main drugs within the non-nucleoside reverse transcriptase inhibitor drug class (efavirenz and nevirapine) appears not to be associated with an adverse risk of CVD,43 although knowledge on effects from longer-term exposure is limited at present. Of note, both non-nucleoside reverse transcriptase inhibitors increase HDL, but not LDL-cholesterol levels.46,47 Finally, whether one or more drugs within the nucleoside reverse transcriptase class also affects risk of CVD remains to be determined, although stavudine is linked to increase in cholesterol levels (Table 68-1).48 Lipodystrophy is associated with dyslipidemia and insulin resistance (see Chapter 71 Chapter 72–Chapter 73), but IHD is not seen more frequently in patients with lipodystrophy.7
Clinical Evaluation and Interventions Aimed at Reducing Risk of CVD
Until the time when such studies have been completed, the most reasonable approach nevertheless appears to be utilizing the strategies applied to the general population.49
The interventions consist of lifestyle changes and medical interventions. A large body of literature exists concerning the general population, documenting the benefits derived from four types of intervention: blood-pressure reduction in patients with hypertension, reduction of LDL cholesterol, use of acetylsalicylic acid (ASA) in patients with prior CVD, and smoking cessation. In Table 68-2 the relative benefit that can be expected from using each of these interventions, either alone or in combination, is depicted.
Table 68-2 The Expected Benefit From Interventions Aimed at Reducing Risk of Ischemic Heart Disease (IHD) and Stroke
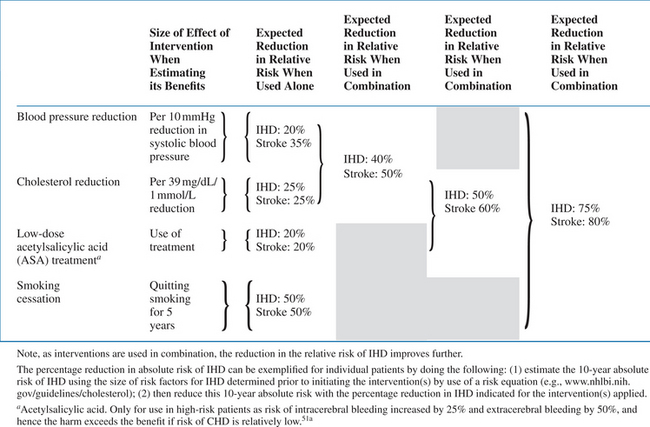 |
Source: Summary of the entirety of randomized controlled trials that have assessed these four interventions. Modified from MacMahon.51
Smoking can be expected to be associated with the largest reduction in risk. Normalization of blood pressure reduces the risk of stroke more than the risk of IHD. Combination of the interventions results in added benefit (Table 68-2).50,51 When all interventions are combined, the risk of CVD may be reduced by as much as 80%. For example, in a male smoker with a prior myocardial infarction, hypertension and dyslipidemia leading to a projected 30% risk of a new IHD in the next 10 years; this risk can be reduced to 6% by appropriate interventions. Of note, the net benefit (in absolute terms, in the example, 3026% = 24% over a 10-year period) from these interventions obviously depends on the patient’s a priori absolute CVD risk. For example, if the a priori risk was only 5% risk of IHD in the next 10 years, an 80% reduction constitutes a 1% risk of IHD (i.e., a 4% absolute risk reduction). Whereas lifestyle interventions have no apparent disadvantages (except impairment of quality-of-life which adversely affects adherence to the intervention), medical interventions are all associated with potential harm. Hence, a guiding principle in CVD prevention is to assess the individual person’s absolute CVD risk, focusing the most aggressive interventions on those with the largest expected benefit in absolute terms.
A prediction of absolute risk is usually focused on the risk of IHD, as several so-called ‘risk equations’ exist that determine this risk. It is currently debated as to how to apply these risk equations in HIV, since none of them have been appropriately validated in this population and do not include the entirety of risk factors for CVD identified in HIV (most notably, none of them account for exposure to ART).52 One study40 has compared predicted and observed absolute risk of myocardial infarction in HIV infection, and found that the Framingham equation53 fairly accurately predicted the observed incidence, whereas the ‘Copenhagen risk equation’54 under-predicted the observed risk by 40%. There is an ongoing effort to develop a risk equation specific for HIV infection. Until this work has been completed, use of the Framingham equation to predict the absolute risk of IHD in HIV (see: http://www.cphiv.dk/TOOLS/Framingham/tabid/302/Default.aspx) is recommended.
Practicalities of Using the Interventions
In Figure 68-5 is outlined an algorithm for routine evaluation and interventions aimed at reducing LDL cholesterol in HIV infection, based on modification from the ‘National Cholesterol Education Programme’ (NCEP) III guidelines55 and comparable guidelines in Europe.
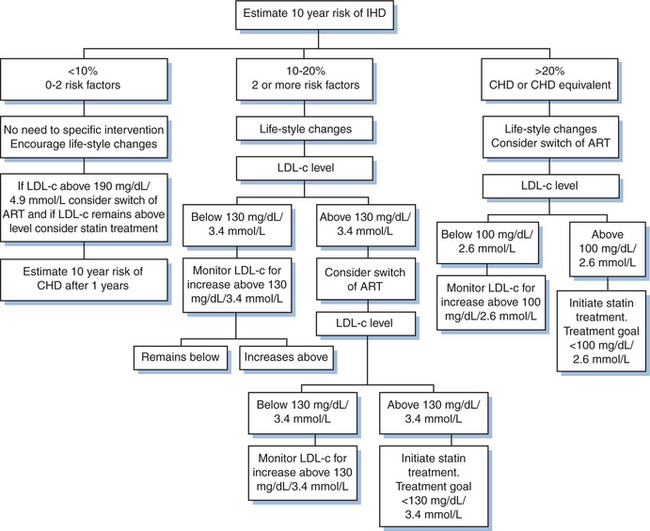
Figure 68-5 Algorithm for the prevention of cardiovascular disease in HIV-infected patients. Estimation of 10-year risk of IHD–use http://www.cphiv.dk/TOOLS/Framingham/tabid/302/Default/aspx. Risk factors: smoking, age (> 45 years for men; 55 years for women), hypertension (well controlled), HDL cholesterol < 40 mg/dL, family history of premature CHD. Lifestyle changes: daily intake of saturated fats < 7% of total calories, daily intake of cholesterol < 200 mg, increase vegetables, and fiber intake, weight reduction and increased physical activity (30–60 minutes per day). Goals for treatment aimed at reducing LDL cholesterol: the LDL level above which the treatment is indicated (note, this level differs depending on the absolute CVD risk). In case the LDL cholesterol cannot be determined because of high triglyceride levels, the nonHDL cholesterol level is calculated (total cholesterol minus HDL cholesterol), and 30 mg/dL (0.8 mmol/L) is added to the levels indicated in the figure.
First, the absolute risk of IHD is assessed and the population is stratified into three main categories of risk: low (<10% risk of IHD over the next 10 years), intermediate (10–20% risk), and high (>20% risk). The proportion of contemporarily followed HIV-infected patients with a 10-year risk of IHD exceeding 10% (intermediate to high risk) is 10–15% and this percentage is projected to be doubled by 2015, merely due to aging of the population.41
Lifestyle interventions are the first interventions to be considered (Fig. 68-5). The aggressiveness is intensified as the absolute risk increases, although all patients should be targeted. Exercise, quitting smoking, and diet modifications are the key components to consider.
Suggested key elements of the daily diet are:
All adults are recommended at least 30 min of moderately intensive physical activity daily.29 Management of the addiction to nicotine56 as well as the commitment of the patient to cease smoking are key determinants in successfully quitting this lifestyle. The diet modifications and the intensification of exercise may reduce LDL-cholesterol levels.
A dilemma for rational management of CVD in HIV infection is, how most appropriately to medically intervene in case of elevated LDL cholesterol that is not correctable by lifestyle interventions. The definition for an elevated LDL-cholesterol level requiring intervention depends on the absolute CVD risk of the individual patient (Fig. 68-5). There are two principal possibilities, namely switch in components of ART known to enhance LDL-cholesterol levels or providing lipid-lowering medication.56,57
Switch in ART is preferable if it is plausible that the new regimen will result in better metabolic control58,59 and if the intended switch(es) do not render the patient more susceptible to virological failure due to archived viral resistance or inferior intrinsic antiviral activity of the new drug combination planned to be used. The treatment history and previously performed resistance tests can be used to assess the presence of archived resistance, whereas the reader is referred to the Chapters in section 3, Anti-retroviral therapy for information on differences in intrinsic antiviral activity of various combinations of anti-HIV drugs.
The lipid levels vary in the background population (as they do in patients infected with HIV receiving ART)60 due to genetic and diet differences. Switch in components of ART can only be expected to reduce lipids in patients where ART increased the levels in the first place. In an attempt to separate preexisting from drug-induced dyslipidemia, screening patients for metabolic abnormalities prior to commencing ART is recommended. Switches are especially encouraged in case the patient is receiving a protease inhibitor. Of note, in the background population, there is a small increase in LDL and a decrease in HDL cholesterol associated with increasing age61 which should be incorporated into considerations when changes are assessed in lipids in individual patients over many years.
Statins can be used in the case of elevated LDL cholesterol. Of note, drug–drug interactions may arise because certain statins and anti-HIV drugs are metabolized via the same pathway (see below), and the choice of statins is reduced (see below). The level of LDL cholesterol above which statin treatment is indicated varies according to the patient’s absolute risk of IHD (Fig. 68-5). The aim of using the statin is to reduce the level of LDL cholesterol below this level. If statin monotherapy is not sufficient to reduce LDL-cholesterol levels sufficiently, combination therapy with bile acid resins (e.g., cholestipol, cholestyramin), ezetimibe, or nicotinic acid can be considered.62 Ezetimibe adds additively to the LDL-reducing ability of statins,63 but the potential for side effects and drug–drug interactions suggest that combination therapy should be reserved for high-risk patients only.
If the triglyceride levels are high, LDL-cholesterol levels cannot be calculated. In such situations, make sure that lipids are measured in fasting (at least 8 h without food) condition as food intake predominantly adversely affects triglyceride levels.64 Cessation from long-term massive alcohol-intake, triglyceridemia-inducing drugs (discussed earlier), and certain antiretroviral drugs (most pronouncedly lopinavir, ritonavir,65,66 and stavudine)48 tend to reduce triglyceride levels. If the triglyceride level cannot be reduced sufficiently, non-HDL cholesterol levels can be used as a surrogate for LDL-cholesterol levels. The target non-HDL cholesterol level (total cholesterol minus HDL level) is 30 mg/dL (0.8 mmol/L) higher than the corresponding LDL cholesterol target (Fig. 68-5).
Fibrates can be considered in situations where triglyceride levels are high and constitute the major lipid abnormality and where alcohol cessation, diet restriction, other nonmedical interventions, and possible substitutions of drugs that may increase triglyceride levels are not able to improve the situation. One definition of ‘high’ triglyceride levels is when the plasma concentration of triglycerides exceeds 8 mmol/L (700 mg/dL), except for patients with a history of pancreatitis where interventions should be considered if plasma triglyceride levels exceed 5 mmol/L (440 mg/dL). The clinical benefit from using fibrates in HIV infection is however questionable. First, the level of triglycerides in plasma appears not to be a strong independent risk factor for CVD in HIV infection and in the general population (as discussed earlier). Second, the overall clinical benefit from fibrate treatment is not apparent from the review of three randomized intervention studies of non-HIV-infected populations.67–70 Third, although more than 20% of HIV-infected patients have increased trigly-ceride plasma levels, the rate of pancreatitis is low and associated with traditional risk factors71 (Lundgren, personal communication), and hence it is questionable whether the risk of pancreatitis is increased in HIV-infected persons with high triglyceride levels. Finally, triglyceride levels in HIV are often due to drugs or lipoatrophy, and only rarely associated with obesity and the metabolic syndrome, which is often the case in the background population. Only in very selected cases is it recommended to combine a statin and a fibrate because of risk of drug–drug interaction, and if this is done, fenofibrate appears most suitable.72,73
Both statins and fibrates have been demonstrated to reduce LDL cholesterol and triglycerides, respectively, in HIV-infected persons on ART.74–77 These studies serve as proof-of-conceptthat ART-induced dyslipidemia is medically correctable, although they also point out that the desired optimal effect (reduction to below levels indicated in Table 68.5) is rarely met.
Table 68-5 Clinically Significant Drug Interactions Between Antiretroviral Drugs and Drugs Used to Prevent or Treat Cardiovascular Disease
| Drugs Used to Treat Cardiovascular Disease | Non-Nucleoside Reverse Transcriptase Inhibitors | Protease Inhibitors |
|---|---|---|
| Acetylsalicylic acid | Use | Use |
| Anti-arrhythmics | ||
| Digoxin | Use | [Anti-arrhythmics]p ↑ |
| Disopyramide, lidocaine, mexiletine, | [Anti-arrhythmics]p↓ | [Anti-arrhythmics]p ↑ |
| Amiodarone, flecainide, propaferone, | [Anti-arrhythmics]p↓ | Contraindicated |
| Beta-blockers | ||
| Atenolol, bisoprolol, propranolol | Use | Use |
| Carvedilol | Unknown | Unknown |
| Calcium channel antagonists (Ca2+ ch. antag.) | ||
| Amlodipine, diltiazem, nicardipine, nifedipine, nisoldipine, verapamil | [Ca2+ ch. antag.]p ↓ | [Ca2+ ch. antag.]p ↑ |
| Statins | ||
| Fluvastatin, pravastatin, rosuvastatin | Use | Use |
| Atorvastatin | [Atorvastatin]p ↓ | [Atorvastatin]p ↑ |
| Lovastatin, simvastatin | Contraindicated | Contraindicated |
If the plasma concentration of the first drug is reduced after co-administration with the HIV drug, higher than normal doses of the first drug may be required to accomplish the desired effect.
If the plasma concentration of the first drug is increased after co-administration with the HIV drug, lower than normal doses of the first drug may be required to accomplish the desired effect.
Source: www.hiv-druginteractions.org.
Many clinics have introduced screening for insulin resistance by use of a 2-h oral glucose tolerance test. Antiretroviral drugs can induce insulin resistance78,79 and overt diabetes, and lipodystrophy is associated with insulin resistance.80 Other than addressing these issues, the clinical implications from detecting an abnormal oral glucose tolerance test not fulfilling the criteria of diabetes are limited for CVD prevention. The insulin sensitizers (roziglitazone has been most extensively studied) improve determinants of insulin resistance but also aggravate dyslipidemia81–84 and have other adverse effects and should not be used routinely. However, the oral glucose tolerance test is well suited to identify patients at excess risk of prestages of diabetes or overt diabetes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree