14 Cancers of the skin
Cutaneous melanoma
Aetiology
Pathology
There are four histological variants of cutaneous melanoma:
Other forms of melanoma include ocular, mucosal and vulval (p. 236).
The histology of melanomas is very variable depending on the specific subtype and primary site. Many of the features assessed by histology have prognostic value (Box 14.1). In addition to these the pathologist should report the type of melanoma, greatest thickness, radial or vertical growth phase, excision margins and immunohistochemical stains. Immunohistochemical stains used in melanoma include S100 (the most frequently used but also stains benign melanocytes), HMB-45, Mitf, MART-1 and tyrosinase.
Initial assessment
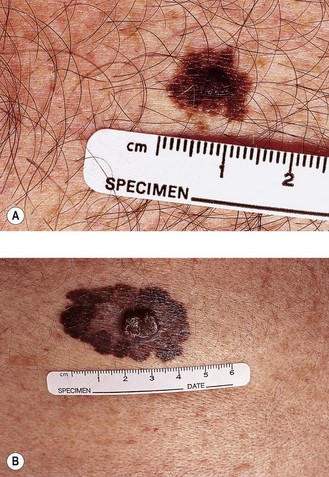
Initial assessment of patients with suspected melanoma includes detailed examination and a clinical photograph of the lesion, full skin examination and examination for lymphadenopathy and hepatomegaly. Clinical signs of melanoma include itching, bleeding, ulceration or changes in a pre-existing mole. ABCDE features (Figure 14.1) help to distinguish early melanoma from a benign mole:
Investigations
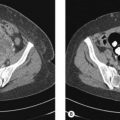
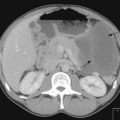
In the absence of clinical evidence of metastatic disease in regional lymph nodes at presentation, there is no indication for any staging investigations. In patients with thick primary tumours (>4 mm), CT scan may be useful prior to rule out metastatic disease. Studies show that a routine chest X-ray revealed metastasis in only 0.1% whereas 15% showed false positivity. Similarly CT scan showed metastasis in 1.3% while false positivity was 16%.
Staging
Breslow thickness, ulceration and mitotic count are most important prognostic factors of localized disease whereas Clark level is an independent prognostic factor for melanoma of <1 mm thickness (Box 14.1). Staging is by TNM AJCC system and is related to prognosis (Table 14.1). M1a includes distant skin, subcutaneous or nodal metastases with a normal LDH. M1b is lung metastases with a normal LDH and M1c is with any other metastases or with a raised LDH level.
Table 14.1 TNM staging & 5-year survival of cutaneous melanoma
| Staging | 5-year survival (%) |
|---|---|
| Stage IA | 95 |
| T1aN0M0 ≤1 mm thickness without ulceration and mitosis <1/mm2 | |
| Stage IB | 91 |
| T1bN0M0 ≤1 mm thickness with ulceration or mitosis ≥1/mm2 | |
| T2aN0M0 1.01–2 mm thickness without ulceration | |
| Stage IIA | 77–79 |
| T2bN0M0 1.01–2 mm thickness with ulceration | |
| T3aN0M0 2.01–4 mm thickness without ulceration | |
| Stage IIB | 63–67 |
| T3bN0M0 2.01–4 mm thickness with ulceration | |
| T4aN0M0 >4 mm thickness without ulceration | |
| Stage IIC | 45 |
| T4bN0M0 >4 mm thickness with ulceration | |
| Stage IIIA | |
| T1-4aN1aM0 Micrometastasis* in 1 node | 70 |
| T1-4aN2aM0 Micrometastasis in 2–3 nodes | 63 |
| Stage IIIB | |
| T1-4bN1a/2aM0 | 50–53 |
| T1-4aN1bM0 Macrometastasis** in 1 node | 46–59 |
| T1-4aN2bM0 Macrometastasis in 2–3 nodes | 46–59 |
| T1-4aN2cM0 in-transit metastases/satellites without nodes | |
| Stage IIIC | 24–29 |
| T1-4bN1b/2b/2cM0 | |
| Any T N3M0 metastasis in >3 nodes or matted nodes or in-transit or satellites with metastatic nodes | |
| Stage IV | |
| Any T, any N, M1 distant metastasis | 7–19% |
*micrometastases after sentinel node biopsy; **macrometastases are clinically detectable pathologically confirmed nodes.
Management
Non-metastatic disease
Adjuvant treatment
No trial has shown a survival benefit for adjuvant chemotherapy in localized malignant melanoma. Due to the activity of immune treatment in advanced melanoma several trials have examined the role of interferon and vaccines in the adjuvant treatment of melanoma. A pooled analysis of three ECOG studies showed that adjuvant high dose interferon improves relapse-free – survival (about 10% at 5- years) but not overall survival in patients with a high-risk resected melanoma. This group included patients with ≥4 mm thick with no nodal involvement and melanoma involving regional nodes or in-transit metastasis. However, high dose interferon is associated with a number of side effects such as acute constitutional symptoms, chronic fatigue, headache, weight loss, nausea, myelosuppression and depression and most patients will require dose modifications during treatment.
Management of positive nodes
Patients with metastatic lymph node disease are treated with regional node dissection if feasible and up to 13–59% of these patients do not develop metastatic disease. There is a significant risk of lymphoedema (especially with groin dissections) and patients should wear compression stockings for many months. Some trials are examining the role of adjuvant treatment, such as bevacizumab, after groin dissection. A randomized study has evaluated the role of postoperative radiotherapy (48 Gy in 20 fractions) in patients with a high risk (>25%) of regional recurrence after lymphadenectomy. The two-year lymph node field relapse-free rate was significantly improved with adjuvant radiotherapy compared with observation (82 vs. 65% HR 0.47, 95% CI 0.28–0.67); but without no improvement in 2-year overall survival. The long term toxicity of radiotherapy yet to be reported.
Management of metastatic disease
Role of radiotherapy
Palliative radiotherapy is useful in bone metastasis and spinal cord compression not amenable to surgical decompression (p. 328). Skin metastases which are rapidly enlarging and about to ulcerate can also be treated with palliative radiotherapy.
Chemotherapy and other systemic treatments in metastatic disease
Dacarbazine is the standard intravenous chemotherapy drug for metastatic melanoma with a response rate of 10–20% and a median duration of response of 3–6 months. It is given 3–4 weekly at doses of 850–1000 mg/m2 with nausea as the main side effect. Temozolamide is an oral analogue of dacarbazine and crosses the blood–brain barrier but is more expensive and has no improvement in response rates compared with dacarbazine (Table 14.2). There is no evidence that combination chemotherapy regime is superior to single agent drugs in terms of response rates or survival.
Table 14.2 Response rates (%) for single agents in metastatic melanoma
| Agent | % Response rate (CR + PR) |
|---|---|
| Dacarbazine | 20 |
| Temozolamide | 21 |
| Carmustine (BCNU) | 18 |
| Lomustine (CCNU) | 13 |
| Cisplatin | 23 |
| Carboplatin | 16 |
| Vinblastine | 13 |
| Paclitaxel |