Tumor Cells and Cell Extracts
In phase 2 trials, a vaccine composed of three allogeneic cell lines and BCG (Canvaxin) was shown to produce durable tumor regression in a small subset of patients with unresectable in-transit and metastatic melanoma, resulting in increased survival compared to historical case-matched controls for both stage III and fully resected stage IV disease.
11,
12 Two large randomized trials of 1,160 stage III and 496 stage IV patients, all of whom had surgery to remove all known disease, compared the three cell line vaccine to BCG plus placebo, and final results were presented in abstract form. Surprisingly, survival in the BCG plus placebo arm was better in both studies and reached statistical significance for the stage III patients (
P = 0.047). Overall survival (OS) in the BCG plus placebo arms was higher than expected compared to other randomized trials and reached 40% at 5 years in the stage IV no excellence of disease (NED) patients. The data cannot exclude the possibility of a beneficial effect from the BCG and/or a harmful effect of the cell-based vaccine; in the latter case possibly by inducing a population of T-regulatory cells that inhibit immune surveillance and thus accelerate tumor progression (see below).
A novel method to harvest tumor-specific peptides from individual tumors was discovered during the decade of the 1990s when Srivastava et al. showed that stress proteins such as gp96 and HSP70 bind to antigenic peptides and that the HSP/peptide complexes could be used in preclinical vaccine studies to illicit tumor-specific immunity, especially against micrometastatic disease.
13 A phase III adjuvant study was performed in renal cell carcinoma (RCC) patients with the HSP vaccine product harvested from nephrectomy specimens. This study, which enrolled over 800 patients and took more than 7 years to complete, failed to demonstrate a benefit of vaccination in OS or relapse-free survival.
14 A post-hoc analysis of patient data showed that approximately 40% of patients
identified as having recurrent disease actually had residual disease postoperatively and should not have been enrolled in an adjuvant study. A secondary analysis of the data using a newer staging system of early-stage disease suggested a significant improvement in event-free survival (EFS) in vaccinated patients.
A similar phase 3 trial in metastatic melanoma compared tumorderived HSP gp96-peptide complexes (vitespen) to a physician’s choice of standard therapy including temozolomide, dacarbazine, or interleukin-2.
15 Four hundred and fifty patients with metastatic disease were screened, and 322 were randomized 2:1 (215 versus 107) to receive vitespen or standard treatment. However, only 133 patients received a dose of the vaccine, and only 86 received standard treatment on trial. There was no difference in survival between arms when analyzed by intent to treat or in patients receiving treatment.
A phase II study of HSP/peptide vaccination plus IL-2 was performed in patients with metastatic RCC who underwent nephrectomy.
16 Of 60 patients, there were two complete response (CR) and two partial response (PR), and it was concluded that this approach was not an improvement compared with IL-2 treatment alone. Overall, data from trials of unmanipulated whole tumor cells or tumor cell extracts have been disappointing, and it is unclear at this time whether this vaccine approach will be more effective if used as part of combination vaccine regimen (i.e., prime/boost) or in combination with modulators of costimulatory pathways.
Genetically Modified Tumor Cells
Based on the preclinical studies of Dranoff et al.,
6 a phase I adjuvant vaccine study was performed in pancreatic cancer patients who were eligible for potentially curative pancreaticoduodenectomy.
17 The vaccine utilized escalating doses of two pancreatic cancer cell lines PANC 10.05 and PANC 6.03 that had been stably transfected with a GM-CSF expression vector. Six to eight weeks after surgery,
12 of 14 patients still in remission received the priming vaccination, followed by a 6-month course of chemotherapy/radiation. The six patients who were still in remission after adjuvant therapy received an additional six vaccinations at 1-month intervals. There was evidence of antitumor immunity in three of six tested patients as determined by delayed-type hypersensitivity (DTH) response to autologous tumor cells, and these three patients experienced disease-free survival (DFS) of greater than 25 months.
A subsequent study was performed in pancreatic cancer patients with at least one measurable lesion and a Karnofsky performance status (KPS) of greater than 70.
18 The first 30 patients received 6 monthly intradermal injections with the two GM-CSF-secreting pancreatic cancer cell lines (5 × 10
8 total cells split over 4 to 8 sites). The second group of 20 patients received the same vaccination, but it was preceded by a single injection of cyclophosphamide at 250 mg/m
2. Median survival was 2.3 and 4.3 months for the two cohorts, and there was a modest increase in reactivity to mesothelin peptides in cohort 2. Whether cyclophosphamide administration contributed to the improved immune responses, perhaps by inhibition of CD4
+CD25
+ Tregs,
19 remains speculative.
A multicenter study of non-small cell lung cancer (NSCLC) patients with autologous tumor modified by GM-CSF adenovirus demonstrated complete tumor responses in 3 of 33 advanced disease patients. However, GM-CSF secretion by the modified tumor cells (GVAX) was variable, and in many cases, it may have been too low to mobilize DC for antigen presentation.
20 In a follow-up trial, the K562 erythroleukemia cell line was engineered to secrete high levels of GM-CSF, and this was injected along with autologous tumor cells to provide a bystander GVAX effect.
21 Tumor was harvested from 86 stage III/IV NSCLC patients (ECOG 0-2) yielding 76 vaccines of which 49 were actually given to patients. No tumor responses were observed despite robust vaccine site reactions due to high levels of GM-CSF secretion, and DTH responses to autologous tumor cells occurred in a minority of patients.
Eighty patients with metastatic hormone-refractory prostate cancer were vaccinated with PC-3 or LNCaP prostate cancer cell lines that were infected with recombinant GM-CSF-expressing adenovirus.
22 The cell dose was escalated to 500 × 10
6 cells as prime and 300 × 10
6 boost administered every 2 weeks for 11 doses. No maximum tolerated dose (MTD) was reached, and patients receiving the higher cell dose had longer median survival (35 versus 20 months) and were more likely to develop antibodies reactive with cell line extracts. PSA stabilization was observed in 19% of patients and one patient had greater than 50% PSA decline. Two phase III trials of this vaccine failed to show a survival benefit.
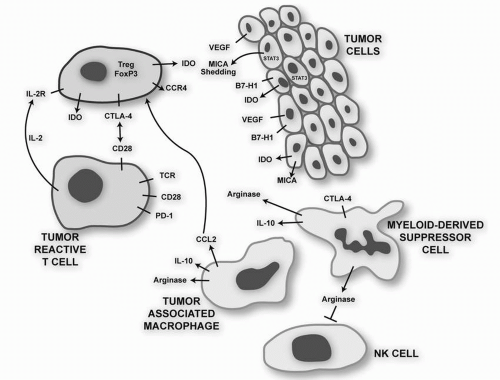
As methods for breaking tolerance to tumor antigens improve, the response of patients to previously unknown antigens may provide an important resource of antigenic targets for future immunotherapeutic studies. For instance, it was recently demonstrated that patients with hematologic malignancies (e.g., acute myeloid leukemia [AML]) who had been on trials using GM-CSF-secreting tumor vaccines or those using blockade of CTLA-4 had high serum levels of antibodies specific for protein disulfide isomerases, suggesting that monoclonal antibodies directed against these targets would be interesting research reagents and possibly novel therapeutics.
23 A number of strategies are being evaluated in preclinical studies in an attempt to enhance the antitumor potency that has been missing from GM-CSF tumor cell vaccines tested to date. These include down-regulating CD4
+CD25
+FOXP3
+ Tregs that are often induced by the vaccines, blocking the inhibitory signal delivered by CTLA-4, and overcoming the suppression of innate and adaptive cytotoxic responses mediated by the action of soluble MHC class I chains on NKG2D receptors.
24
Dendritic Cell Vaccines
Dendreon developed a vaccine for patients with hormone-refractory metastatic prostate cancer in which the patient’s peripheral blood cells, obtained via leukapheresis and buoyant density centrifugation (hence containing monocytes, DC, lymphocytes, natural killer [NK] cells, etc.), were incubated for 40 hours with a recombinant fusion protein composed of prostatic acid phosphatase (PAP) cloned in frame with GM-CSF and then infused into the patient three times at 2-week intervals. An initial phase III study in 127 prostate patients comparing Provenge with placebo demonstrated a 41% reduction of the risk of death (
P = 0.01) in vaccinated patients but not EFS (
P = 0.052; 11.7 versus 10.0 weeks), which was the prospective end point of the trial.
25 The Food and Drug Administration (FDA) required a second trial with OS as the end point (IMPACT, Immunotherapy for Prostate Adenocarcinoma Treatment), and results of the study, which were recently presented, demonstrated a significant 22% reduction in death in the vaccinated arm. FDA approval of this vaccine is expected soon.
A randomized trial was also undertaken to compare the activity of adoptively transferred, autologous monocyte-derived mature DC to standard treatment with dacarbazine in patients with metastatic melanoma.
26 DCs were loaded in vitro with 2 to 6 MHC class I-restricted peptides from melanosomal or cancer-testes antigens and with three class II-derived peptides from MAGE-A3, tyrosinase, and gp100. Although not clearly stated, peptide loading of DC was based on the known MHC class I expression of the patient; all patients in the vaccine arm received DC pulsed with the three MHC class II-restricted peptides. The trial was stopped early after 108 patients were accrued and no differences were found between arms for progression-free survival and OS. Objective responses were observed in 5.5% and 3.8% of control and vaccinated patients, respectively.
In vitro-generated autologous DCs from 28 patients with advanced gastrointestinal (GI) malignancies were pulsed with HLA-A2- or HLA-A24-binding peptides from MAGE-3, and patients were vaccinated subcutaneously near axillary and inguinal LN.
27 Each patient received four vaccinations at 2-week intervals consisting of 3 × 10
7 pulsed DC. A few patients had minor tumor responses. CTL responses were tested in only eight patients against pulsed cell line targets, not autologous tumor.
A phase I/II vaccination study was performed using a novel antigen loading strategy in which allogeneic DCs were electrically fused with autologous tumor-derived cells derived from patients with stage IV RCC.
28 Allogeneic DCs were used since in a previous phase I trial, 23 patients with breast or RCC were vaccinated with autologous DC/tumor fusions.
29 This was well tolerated, and immune responses were detected in some patients, but clinical responses were rare. Since it is thought that DC from advanced cancer patients might have an impaired capacity to process and present antigen and may express lower levels of costimulatory molecules,
30 the subsequent trial fused allogeneic DC from normal volunteers
with autologous tumor from the patient. Twenty-four patients were vaccinated with 40 to 100 million cells (three vaccines at 6-week intervals). The fused cells expressed DC markers including HLA class II and costimulatory molecules, and also tumor antigens. Although 10/21 tested patients showed evidence for increased T-cell secretion of IFN-γ in response to tumor lysates in vitro, only 2 of 20 patients had PRs by RECIST criteria.
A phase I/II therapeutic DC vaccination was performed in 27 patients with relapsed/refractory RCC.
31 Autologous PBMC were cultured in GM-CSF and IL-4, and either pulsed with tumor cell lysates and matured, or matured and then pulsed with a panel of HLA-A2-binding peptides from telomerase and survivin, with addition of the pan T-helper peptide PADRE. Autologous antigen-pulsed DCs were injected into inguinal LN under ultrasound guidance, and 2 × 10
6 units of IL-2 were administered subcutaneously. In a subset of patients tested, antigen-specific-T-cell responses were noted using IFN-γ ELISPOT and/or tetramer analysis. There were no tumor responses; 13 of 27 patients had stable disease (SD) for >8 weeks, and one third of patients had SD for greater than 6 months, although a cause and effect relationship with immune reactivity was not proven.
In an attempt to generate CTL directed against p53-expressing breast cancer cells, autologous, in vitro-generated DCs were pulsed with six HLA-A2-binding peptides from p53 and the PADRE helper peptide.
32 Patients received at least six subcutaneous vaccines at weekly or biweekly intervals given with 6 million units of IL-2. One third of patients had SD, and it was noted that after just four vaccines CD4
+CD25
+ Tregs had doubled in number, possibly in response to the IL-2 or the fact that the DC used in the study had not been matured prior to vaccination, a condition that has been associated with tolerance induction.
33The possible inhibitory role of vaccine-induced Tregs was demonstrated in a study in which 18 patients with relapsed, indolent non-Hodgkin’s lymphoma (NHL) were treated with autologous DC (in vitro differentiated with IL-4/GM-CSF), which were cocultured with autologous lymphoma cells, and then matured with TNF-
α.34 The autologous lymphoma cells were heat pulsed to increase HSPs and tumor antigenicity and then killed with UV irradiation. Patients received four subcutaneous vaccines at 2-week intervals. There were three CR (which were of longer duration than previous CR induced by chemotherapy), three PR, and eight patients had SD. Responding patients had a reduction in CD4
+CD25
+FOXP3
+ Tregs and an increase in NK and effector memory cells. There was also evidence for a tumor-specific humoral response. The decrease in Tregs is of special interest given the recent data that NHL cells can recruit Tregs by secretion of chemokine CCL22 and that normal T cells can be induced to differentiate toward a Treg phenotype by the tumor microenvironment, perhaps by TGF-
β secretion by NHL cells.
35,
36,
37There are 500,000 cases of hepatocellular carcinoma (HCC) worldwide, and the annual incidence is on increase in the United States due to chronic hepatitis C infection. Although a modest survival advantage occurs with sorafenib therapy, few therapeutic options are available to treat the tumor and associated field defect. In an attempt to induce CTL directed against HCC, autologous DCs were isolated from 2 hours plastic-adherent PBMC followed by culture in GM-CSF and IL-4.
38 These immature DCs were cultured with lysates from the HepG2 HCC cell line, and after phagocytosis, the DCs were matured by culture in TNF-
α. Thirty-five patients who were not candidates for surgery or liver-specific loco-regional therapies received a maximum of six vaccines administered every 3 weeks. Of 25 patients who received at least three vaccine infusions, 28% had SD for greater than 3 months, but only one patient of 25 evaluable had a radiologic PR. In 4 of 17 patients with a pretreatment alpha fetoprotein (AFP) >1,000 ng/mL, there was a decrease to less than 30% of baseline. Median survival of 35 treated patients was 165 days. In a small number of patients, T-cells responses were evaluated by IFN-
γ ELISPOT, which showed some evidence of immune induction, but the responses were in many cases short lived or equal to negative controls.
DNA, Viral, and Bacterial Vaccines
Another novel immunologic concept from the 1990s was the finding that direct injection of naked plasmid DNA encoding protein antigens into skeletal muscle resulted in expression of the antigen and the induction of humoral and cellular immunity that had antitumor activity in murine models.
39,
40 In a recent study,
41 15 patients with biopsy-proven HPV 16-positive, cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3) received intramuscular injection with 0.5, 1.0, or 3.0 mg of a plasmid expressing a fusion protein encoding a mutated E7 (unable to bind Rb; transformation defective) fused to HSP70 to increase DC uptake and a signal peptide for secretion from the cell that took up and expressed the injected DNA. There was no dose-limiting toxicity (DLT). Patients then underwent standard therapeutic resection of the cervical squamocolumnar junction at day 105. Of the nine patients receiving the 3-mg dose, three had complete disappearance of the CIN 2/3 lesion upon histologic examination of the resected areas. However, these results show little difference from the 20% to 25% spontaneous remission rate of HPV16-associated CIN 2/3 lesions that occurs after diagnostic biopsy.
42 Immune monitoring failed to detect induction of antibody to E7 in any patient, and there was little evidence for specific E7-directed T-cell response as assayed using an IFN-γ ELISPOT assay. Overall, these were disappointing results, but not unexpected given the general poor performance of intramuscular DNA vaccines especially in inducing T-cell immunity. It is widely held that future use of DNA vaccines will require the use of heterologous prime-boost regimens, where the DNA vaccine is used to prime, and a second vaccine method such as antigenloaded DCs or recombinant viral vector is used to boost.
43