Abstract
Cancer-related fatigue (CRF) is a severe and distressing form of fatigue that is often not alleviated by rest. Individuals describe a general sense of tiredness and associated functional deficits. CRF varies according to diagnosis, but patients with central nervous system (CNS) tumors may experience symptoms. Variables that influence the development of CRF are multifactorial, but may be both microscopic and macroscopic in nature. Dysfunction of the CNS to deliver appropriate responses may also result in fatigue.
Several conditions may lead to fatigue in cancer patients, such as disturbances in sleep-wake cycles, anemia, endocrinology disorders, and dietary intake. Medication effects for the treatment of cancer and its associated symptoms should also be considered. Anxiety and depression have both been associated with CRF, as have treatments such as radiation and surgery. Diagnosis is possible with patient-reported outcomes, screening tools, and clinical evaluation. Interventions may include physical and functional modalities, medications, and supportive measures. Understanding of the societal impacts of CRF, specifically as it relates to return to work and the financial implications for individuals and their families, allows for comprehensive approaches to management.
Keywords
Exercise, Fatigue, Mass screening, Patient-reported outcome measures, Return to work
Cancer-related fatigue (CRF) is defined as:
… a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.
Unlike fatigue for individuals without cancer, CRF is more severe and distressing, and less likely to be relieved by rest. Patients describe a general sense of tiredness associated with functional deficits.
Epidemiology
Demographic and Prevalence
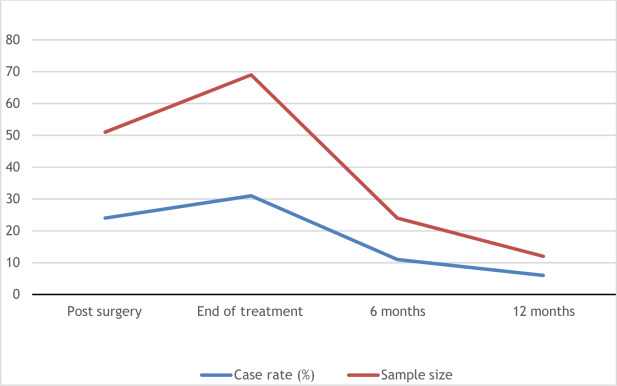
CRF is a common condition, and its prevalence can range from 50% to 100% based on the clinical status of the cancer. The presence of CRF varies according to diagnosis. For instance, individuals with lung cancer experience CRF in 37%–78% of cases, whereas those with breast cancer range from 28% to 91% and prostate can be as low as 15%. However, for some population the presence of CRF can improve over time ( Fig. 12.1 ). For patients receiving active oncologic treatment, moderate to severe fatigue was reported by 45% of individuals and associated with several variables ( Table 12.1 ). In cases for individuals not currently receiving cancer treatment, who are either in complete remission or have no evidence of disease, severe fatigue was noted for 29%. Moderate to severe fatigue was also associated with poor performance status and history of depression.
| Risk Factor | Odds Ratio |
|---|---|
| Strong opioid use | 3.00 |
| Poor Eastern Cooperative Oncology Group (ECOG) performance status | 2.00 |
| Greater than 5% weight loss within 6 months | 1.60 |
| Greater than 10 concurrent medications | 1.58 |
| Lung cancer | 1.55 |
| History of depression | 1.42 |
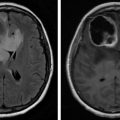
Individuals with central nervous system (CNS) tumors may also experience symptoms. Between 89% and 94% of patients with recurrent malignant gliomas experience fatigue. For primary brain tumors, severity is associated with difficulty sleeping, distress, drowsiness, pain, and weakness. Strong predictors of fatigue include disease status, female gender, and poor Karnosfsky performance status. Disease status is closely associated with severity in women, whereas for men, stronger associations are noted with antidepressant use, opioid utilization, and performance status. In advanced cancer, risk factors may include brain metastases, poor performance status and quality of life (QOL), and reduced ability to perform activities. Prior radiation therapy was associated with less fatigue. However, severity was independently predicted by the presence of brain metastases and poor QOL. CRF can also be present before, during, and after intervention for spinal cord tumors.
Etiology
Pathophysiology
CRF is a multifactorial condition, and variables that influence its development can be both microscopic and macroscopic. At the cellular level, fatigue may originate within the muscle or neuromuscular junction. Factors such as decreased pH, accumulation of lactate, and changes in intra- and extracellular ion concentrations can influence membrane excitability in the muscle, thus causing weakness and fatigue at the synaptic level. Fatigue originating at this level is termed peripheral fatigue. Prevalence for peripheral fatigue may range from 19% to 39%.
However, fatigue may also be the result of ineffectiveness of the CNS to deliver appropriate responses. This central activation failure is also known as central fatigue and may originate from the brain, spinal cord, and nerves. Central fatigue had a greater association with CRF, compared with peripheral fatigue. Causes may include systemic treatments with chemotherapy or focal effects from radiation. Interestingly, between 70% and 96% of individuals who receive either chemotherapy and/or radiation experienced fatigue, whereas those who received surgery alone are at less risk. However, at the molecular level, several other factors can influence the CNS ( Table 12.2 ). Disruptions in the cortical and spinal sensorimotor centers, energy metabolism, and process muscle activation may all lead to reduced physical performance. Many of these may be the result of the tumor itself, as opposed to treatment effects.
| Molecular Factors |
|---|
| Alterations of circadian melatonin secretions |
| Changes in the central nervous system serotoninergic system |
| Disturbances of hypothalamic regulatory circuits |
| Dysregulation of inflammatory cytokines produced by the body or tumors |
| Expression of proinflammatory cytokines used for treatment of cancer |
| Gene polymorphisms for regulatory proteins of oxidative phosphorylation |
| Metabolism of catecholamines |
| Transduction signals in B cells |
Medical Comorbidities
Several conditions may lead to fatigue in cancer patients. Disturbances in sleep-wake cycles are a common occurrence for the cancer patient. For individuals with breast cancer, poor sleep correlates with high levels of fatigue and delayed circadian rhythms result in increased daily dysfunction due to fatigue. Factors that affect circadian rhythms may include disruptions in biologic rhythmicity, mitotic processes of cancer cells, oncologic treatments and their time of day for administration, and QOL. Other factors that lead to insomnia or daytime sleepiness include chemotherapy, pain, psychiatric disturbances, and radiation. Specific consideration should be given to whether an individual has difficulty falling asleep versus staying asleep.
Anemia is often a comorbidity associated with cancer and is found in 40% of cancer patients as well as 90% of those receiving chemotherapy. The causes of anemia may be multifactorial and related to cancer and its treatment, in addition to organ dysfunction ( Table 12.3 ). Cancer patients with lower hemoglobin concentrations may experience decreased ability to work, greater fatigue, and poorer QOL. Improvement in hemoglobin levels correlate with decreased fatigue and better physical functioning.
| Etiology of Anemia |
|---|
| Blood loss |
| Chemotherapy-induced myelosuppression |
| Erythropoietin deficiencies due to renal disease |
| Functional iron deficiency |
| Marrow involvement of tumor |
Endocrinology disorders can be associated with CRF. Specifically, hypothyroidism is the primary factor associated with fatigue. Cytotoxic agents delivered in the treatment of breast cancer can influence thyroid function, which subsequently contributes to both fatigue and decreased physical activity. Considerations should be made for structural abnormalities in the thyroid, incidental thyroid cancer, medications associated with hormone abnormalities, and history of neck or brain radiation. Thyroid dysfunction can also result from radioactive iodine therapy and newer antineoplastic agents used to treat various malignancies.
Dietary intake during and after treatment may play a significant role in fatigue. Nutritional components have an effect at the cellular level ( Table 12.4 ). Poor nutrition may lead to cancer cachexia, which is a form of malnutrition characterized by progressive and involuntary weight loss. Associated with cachexia is depletion of lean body mass and muscle wasting, which can result in fatigue. Patient-specific nutritional programs can reduce or reverse deficiencies and lead to improved performance status. Nutritional interventions may also significantly contribute to QOL improvements.
| Molecule | Cellular Function |
|---|---|
| Carbohydrates and fats | Energy production |
| Proteins | Cellular construction and structural maintenance |
| Water | Muscle turgor and medium for anabolic and catabolic products |
Medication Effects
When considering the treatment of cancer and its associated symptoms, several medication classes have been used to improve functionality and QOL. However, utilization of these agents may have the unintended consequence of fatigue as a side effect. Although pain itself may be a cause, several analgesics can lead to fatigue and drowsiness. Similar proportions of patients with moderate to severe pain had symptoms of moderate to severe fatigue. Both pain and fatigue have been also shown to negatively correlate with emotional functioning, and both along with depression tend to present as a symptom cluster with cancer diagnoses. Unfortunately many of the medications used to treat this cluster can also cause fatigue. Opioid-induced sedation is common and can be managed with additional medications such as methylphenidate and donepezil. Tricyclic antidepressants have been helpful for anxiety and depression but are commonly associated with lethargy and drowsiness. Antihistamines are used for allergic reactions; however, first-generation H 1 -antihistamines readily caused sedation, drowsiness, and fatigue. Newer second-generation H 1 -antihistamines cause less sedation and have improved efficacy. Furthermore, antiepileptic medications, such as gabapentin or levetiracetam, are often used in the brain tumor population to prevent or treat seizures but can cause lethargy and fatigue.
Psychologic Considerations
Fatigue in cancer patients is associated with both anxiety and depression. Depression can subsequently lead to physical and cognitive impairments in brain tumor populations. For individuals with glioma, who have received concurrent radiation, clinical depression commonly presented within the first 6 months of the radiation treatment. Patients with greater functional impairment were at greater risk. The relationship between fatigue and depression is unclear. Depression may be the result of fatigue, or vice versa. Both may also be the result of an alternate but common pathway. However, depression has been shown to be moderately associated with cancer fatigue, as has, to a lesser but consistent extent, anxiety. Benzodiazepines are well known to cause lethargy and fatigue. As noted earlier, fatigue may also result from administration of antidepressant medications, such as tricyclic antidepressants. Careful attention to the emotional status of cancer patients is also important, as fatigue can result from emotional pain and coping mechanisms.
Treatment Effects
Although CRF may be multifactorial in nature, it is important to understand how the treatments directed toward cancer itself may lead to worsening symptoms of fatigue. Surgery can cause physiologic responses contributing to worsening energy levels ( Table 12.5 ). Preoperative levels of fatigue have also been showed to predict the postoperative severity and should be considered during clinical evaluations. Malaise is common after chemotherapy, as well as anemia and neurotoxicity. For patients receiving radiation, anemia, anorexia, chronic pain, diarrhea, and weight loss may result and can negatively influence physical and psychologic components.
| Causes of Physiologic Stressors |
|---|
| Analgesia |
| Anesthesia |
| Immobilization |
| Infection |
| Mood |
Diagnosis
Patient-Reported Symptoms
As cancer survivorship evolves as a major focus of cancer care, so too does CRF. The National Comprehensive Cancer Network (NCCN) recommends that clinicians evaluate for CRF during initial consultation and at all subsequent visits with associated cancer treatments for advanced diseases. Typically, CRF presents with a noticeable decrease in QOL due to physical and psychosocial impairments. Symptoms are disproportionate to levels of exertion and not relieved by rest or sleep. It is often more severe than other forms of fatigue and may persist far after cancer treatment. As a subjective experience, observable behavioral and physiologic levels may be influenced by the individual’s personal understanding and experiences. Further complicating assessment of CRF is that cancer patients may have difficulty reporting the presence and absence of symptoms, as well as severity. Levels of fatigue may fluctuate throughout the day, as well as longitudinally throughout the oncologic spectrum.
Screening
The primary method by which individuals are identified with CRF is screening. Several organizations, including the NCCN, Oncology Nursing Society (ONS), Collaborative Partnership between the Canadian Partnership Against Cancer and the Canadian Association of Psychological Oncology (CPAC/CAPO), and the American Society of Clinical Oncology (ASCO), have published guidelines related to screening and management of CRF. All consistently recommend frequent assessments. Although CPAC/CAPO uses the Edmonton Symptom Assessment Systems (ESAS) as their recommended tool, each organization has other tools that are similarly scaled. The ESAS specifically uses a 0 to 10 scale and rates fatigue as mild, moderate, and severe.
Self-reported measures are being used with more frequency to identify CRF. However, consistency is variable between different tools as it relates to measurements and areas of assessment. Specifically, some tools may evaluate severity, impact on daily functioning combined with severity, or various manifestations of CRF. Unidimensional tools screen for single items that may detect the presence or absence of CRF but may not focus on symptom severity or the personal effects of CRF on the individual. For example, the Visual Analog Fatigue Scale assesses fatigue severity, and the Brief Fatigue Inventory measures severity over 24 h. Multidimensional tools evaluate CRF in a more comprehensive manner, specifically focusing on behavior, cognition, somatic complaints, and affective domains of function. The Multidimensional Fatigue Inventory is a validated tool that emphasizes subjective experiences of fatigue and captures changes over five domains (general, physical, activity, motivation, and mental). The Patient Reported Outcomes Measurement Information System (PROMIS) tool is a new method for screening based on a fatigue questionnaire and short form. With the use of Item Response Theory and computer adaptive testing, the evaluation process can be made both more efficient and precise. Consensus has yet to be achieved regarding which tools may be most efficient and effective. Unidimensional approaches provide quicker but superficial assessments, whereas multidimensional methods are comprehensive but time consuming.
Clinical Evaluation
When evaluating CRF, a focused clinical and physical history is necessary to understand symptoms and their effects on functionality and QOL. Important considerations include disease status, type and length of oncologic treatment, capacity to induce fatigue, and response to interventions. Understanding whether CRF is related to disease progression versus recurrence may affect short- and long-term clinical decision-making. Assessment of current medications and their potential side effects may provide further insight into potential etiologies. Several medical comorbidities may also contribute to fatigue, including anemia, cardiopulmonary issues, endocrine dysfunction, gastrointestinal and hepatic disorders, infection, neurologic impairments, and renal dysfunction. Hypothyroidism may result from certain types of radiation and systemic therapies. In addition to hypothyroidism, endocrinologic conditions such as hot flashes, hypogonadism from advanced cancers, and adrenal insufficiency can contribute to fatigue. Primary evaluation of hypothyroidism includes thyroid-stimulating hormone (TSH) and free T 4 levels. Subclinical hypothyroidism is possible if TSH is normal or mildly elevated while T 4 levels are normal and may be amenable to hormone supplementation. If there is concern for anemia, initial workup would include a complete blood count to evaluate hemoglobin levels and mean corpuscular volume of red blood cells. Microcytic anemia may suggest iron deficiency, and ferritin levels can be ordered to assess iron stores. Low transferrin saturation and increased total iron-binding capacity also indicate iron deficient states. Macrocytic anemias can be due to B12 and folate deficiencies. Serum folate and B12 levels can be assessed, and if low, can be treated with supplementation. Recommendations may also be made to avoid alcohol, which has been known to contribute to these vitamin deficiencies.
A more global review of the physical, emotional, and cognitive status is necessary to understand how these may impair activities of daily living, function, and overall QOL. Several additional factors can contribute to fatigue ( Table 12.6 ). Sleep disturbances may result from mood disorders, and sleep apnea is a possibility, given potential anatomic changes from cancer and its treatment. Evaluation of appropriate sleep hygiene may provide further insights. An understanding of the sleep environment can provide more information into potentially reversible causes. Examples that can be addressed include daytime napping; individual habits; ingestion of foods high in sugar, caffeine or alcohol; deviation from regularly scheduled sleep; and stimulating activities prior to bedtime. Anxiety and depression may also have a role in sleep irregularities.
| Additional Considerations for Fatigue |
|---|
| Activity level |
| Alcohol and substance use |
| Emotional status |
| Hygiene |
| Nutrition |
| Pain |
| Sleep disturbances |
A comprehensive nutritional assessment may provide insight regarding the development of fatigue. For cancer patients, changes in oral intake may lead to weight loss or gain and changes in lean body mass. These in turn can lead to fluid and electrolyte imbalances which may subsequently affect performance status. Several medical issues can cause difficulties with oral diet, including nausea, vomiting, odynophagia, bowel irregularity, and mucositis. Interest in food may decrease for these reasons, which may then lead to loss of appetite and decreased caloric consumption.
Although currently used in a research capacity, imaging may provide novel methods to understand the neurobiology of CRF. Current studies are using noninvasive techniques to evaluate altered neural networks. Functional connectivity magnetic resonance imaging (fcMRI) has identified aberrant brain connectivity patterns for pain, depression, and insomnia. Using either a seed-based approach or independent component analysis, blood oxygen level dependence signals can be used to display further resting-state connectivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree