Cancer of the Liver and Hepatobiliary Tract
 LIVER CANCER
LIVER CANCER
Hepatobiliary malignancies include hepatocellular carcinoma (HCC), gallbladder cancer, intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and rare neoplasms such as sarcoma and hepatoblastoma.1 There were an estimated 696,000 deaths caused by liver cancer in 2008.2 Liver cancer caused 1.2% of total death throughout the world in 2008. The male-to-female sex ratio was about 2.2 to 1.3 People who live in eastern Asia, middle Africa, and western Africa have higher incidence of liver cancer than those who live in developed countries.4
Patients with liver cancer usually are asymptomatic except those symptoms related to their chronic liver disease. Clinical symptoms are associated with advanced disease; the prognosis is dismal, with a 5-year survival of 0% to 10%. Only one of five patients was amenable to curative resection in the past.5,6 With the implementation of screening programs with α-fetoprotein (AFP) and ultrasonography, improvement of surgical technique, and liver transplantation, the resection rate can be increased to 30% to 50%.7,8 The primary curable treatment of hepatobiliary malignancies remains surgical resection, but most patients had inoperable or unresectable disease at diagnosis. For patients with unresectable disease, modalities such as transplantation, chemoembolization, local ablation, systemic chemotherapy, and molecular target therapy were taken into consideration.
 HEPATOCELLULAR CARCINOMA
HEPATOCELLULAR CARCINOMA
Topographic Anatomy
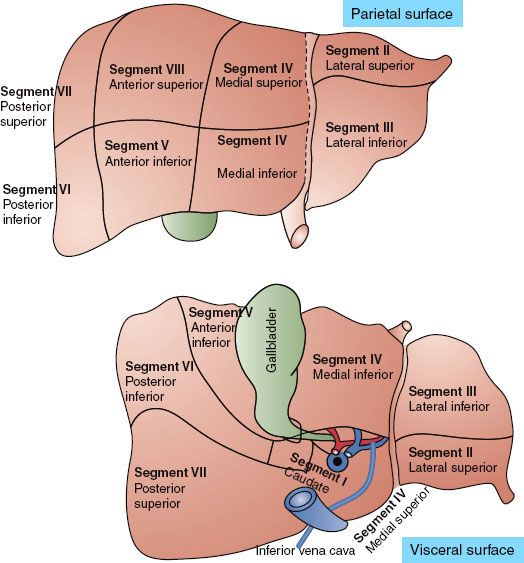
The liver is the largest solid organ in humans. Traditionally it is divided into left and right lobes separated by the falciform ligament. The surgeon needs to understand the spatial relationship of a tumor to the hepatic vascular system preoperatively to determine resectability. Fortunately, progress in imaging techniques has made segmental division of the liver based on the anatomy of portal and hepatic veins feasible. The most common segmentation scheme proposed by Couinaud divides the liver parenchyma into right and left liver with four segments each (Fig. 60.1). The left part of the liver consists of the caudate lobe (segment I), lateral segment (segments II and III), and medial segment (segment IV). The anatomic landmark between the medial segment and lateral segment is drawn between the gallbladder and inferior vena cava (the falciform ligament). The right part of the liver comprises the anterior segments (segments V and VIII) and posterior segments (segments VI and VII). The anatomic landmark that separates the anterior from the posterior segment is the right hepatic vein; the anatomic landmark that divides the anterior segment from the left medial segment is the middle hepatic vein. No good anatomic landmarks exist that further divide the anterior and posterior segments into superior and inferior subsegments.9
Dynamic computerized tomography is very useful in distinctly defining segmental anatomy because the portal vein, hepatic vein, and inferior vena cava can be opacified at the same time.10,11
Epidemiology
HCC is the most frequent primary cancer of the liver and ranks as the fifth most common cancer in the world and the third most common cause of cancer mortality.12 The age-standardized rate of incidence of liver cancer in 1990 worldwide was 14.7 per 100,000 men and 4.9 per 100,000 women.13 The male-to-female ratio was 3 to 1. The highest incidence rate is seen in the male population of South Korea, China, Gambia, and Senegal (28.5 to 48.8 per 100,000 populations).12 In low-risk areas such as Canada, Columbia, and the United Kingdom, HCC occurs in only 1 to 3 persons per 100,000.12 But the incidence rates of primary liver cancer have a trend of decrease among Chinese population in Hong Kong, Shanghai, and Singapore.12 In contrast, the incidence rates in some low-rate areas such as United States, United Kingdom, and Australia increased. The incidence of HCC approximately doubled between 1976 and 2000 in United States.14 The most likely reason for this rising incidence is related to a great prevalence of hepatitis C infection.14
Risk Factors
HCC is clearly associated with hepatitis B (HBV) and hepatitis C (HCV) viral infections and chronic liver disease. With persistent HBV infection, the relative risk of incidence of HCC could be 223.15 The risk of HCC is even higher in patients who are HBeAg (e antigen) positive compared with those who are HBeAg negative.16 As high as for HBV, the relative risk of HCC among persons with chronic HCV infection and cirrhosis is also approximately 100 times the risk of uninfected persons.17 There also exists a synergistic interaction between the two viruses to cause HCC.18
Other chronic liver-cell injury is also associated with the development of HCC. Chemical injury induced by ethanol, nitrites, hydrocarbons, solvents, organochlorine pesticides, primary metals, and polychlorinated biphenyls has been implicated.19,20 Ethanol is the most common culpable chemical agent and is thought to produce HCC through the development of liver cirrhosis or to play the role of a co-carcinogen. Chronic alcohol use of greater than 80 g per day for more than 10 years increases the risk for HCC approximately fivefold. In patients with HCV, alcohol use doubles the risk of HCC.21 Environmental toxins, including aflatoxin, contaminated drinking water, and betel nut chewing, may also be associated with the pathogenesis of HCC. Aflatoxins, well-known hepatotoxic agents, are produced by the fungi Aspergillus flavus and Aspergillus parasiticus. Aflatoxin could contaminate corn, soybeans, and peanuts. High rates of dietary aflatoxin intake have been associated with HCC.22,23 There is a synergistic interaction on HCC between chronic HBV infection and aflatoxin exposure.18 The risk of HCC increases dramatically when both factors are concurrently present. Patients with hereditary liver disease, such as hemochromatosis, Wilson disease, hereditary tyrosinemia, and type I glycogen storage disease, are at high risk of developing HCC.18,24 The common mechanism of developing HCC may be related to chronic injury and inflammation of the liver.
FIGURE 60.1. Segmentation schemes of the liver based on Couinaud’s proposal.
Prevention
Because the development of HCC is highly related to the viral hepatitis and chemical injuries, avoidance of viral infection and chemical exposure may be helpful in prevention of HCC. The HBV vaccine has been available for decades. Universal HBV vaccination in Taiwan has showed a significant decrease of incidence of HCC (from 0.70 to 0.36 per 100,000) in children aged 6 to 14.25 Because the development of HCV vaccination is difficult, the strategies to prevent HCV infection include blood screening, the use of disposable needles and syringes, the adoption of universal precaution for health care workers, and timely treatment of chronic HCV infection with interferon-alpha.26 Meta-analysis suggests that long-term interferon-alfa treatment may prevent the development of HCC in HCV-infected individuals. The preventive effect is more evident among sustained responders to interferon.27
Surveillance
Surveillance for patients with recognized risk factors remains controversial. There is only one randomized controlled trial of surveillance versus no surveillance that has shown a survival benefit,28 and other nonrandomized studies also showed survival benefit of surveillance in high-risk groups.29,30 But when taking the cost into consideration, some authors questioned whether adopting a surveillance policy toward HCC should rely on the prevalence of the disease and the resources of a particular country.30 According to the practice guideline of American Association for the Study of Liver Diseases (AASLD), surveillance is deemed cost-effective if the expected HCC risk exceeds 1.5% per year in patients with hepatitis C and 0.2% per year in patients with hepatitis B. Patients with hepatitis B virus, hepatitis C virus, and autoimmune hepatitis are candidates for surveillance. Current recommendations for surveillance according to the AASLD include:
1. Surveillance for HCC should be based on ultrasonography.
2. AFP alone should not be used for screening because of a lack of adequate sensitivity and specificity for effective surveillance and for diagnosis.
3. Patients should be screened at 6- to 12-month intervals.
4. The surveillance interval does not need to be shortened for patients at higher risk of HCC.31,32
Clinical Presentation
HCC in the early stage is asymptomatic; it is generally detected by elevation of AFP or ultrasonography screening or is an incidental finding in searching for other conditions such as chronic liver disease.33 Even in the advanced stage, some patients are still asymptomatic. Patients with symptoms usually suffer from chronic hepatitis and liver cirrhosis. Clinical symptoms include general fatigue, poor appetite, ascites, jaundice, upper gastrointestinal bleeding, splenomegaly, dilated abdominal veins, palmar erythema, gynecomastia, testicular atrophy, leg edema, and weight loss. Tumor-related symptoms include palpable mass in the upper abdomen (hepatomegaly), acute onset of pain (hemorrhage from tumor rupture), and dull pain in the right upper quadrant of the abdomen, abdominal fullness, low-grade fever, obstructive jaundice, and splenomegaly.34 Very few individuals with HCC present initially with metastatic disease involving extrahepatic organs such as lungs, bone, adrenal glands, pancreas, and neck lymph nodes.35
Diagnostic Workup
In a patient who is suspected of having HCC, the clinical history frequently includes a history of hepatitis, jaundice, blood transfusion, use of intravenous drugs, or exposure to aflatoxins. A family history of hepatitis or hemochromatosis is also an important indicator.36 Details concerning alcohol abuse and job descriptions related to industrial exposure to possible carcinogenic agents are also helpful. The physical examination should include a search for signs of underlying liver disease such as jaundice, ascites, ankle edema, spider angioma on the anterior chest wall, palmar erythema, splenomegaly, increasing abdominal girth, and weight loss. Evaluation of the abdomen for liver size, existence of tumor masses, tenderness, and abdominal bruits should also be performed.
Blood tests should include serology for HBV and HCV, and AFP. If HBV or HCV serology is positive, quantitative HBV DNA or HCV RNA should be obtained.37 Evaluation of hepatic functional reserve includes prothrombin time, activated partial thromboplastin time, and serum albumin. Platelet, red cell, and white blood cell counts also should be obtained to look for simultaneous existence of portal hypertension and hypersplenism from liver cirrhosis. Fifteen-minute retention rate of indocyanine green (ICG) before treatment is useful for determining resectability or the feasibility of radiation.38 The diagnosis of HCC would be included in the differential diagnosis for a patient with underlying liver disease such as cirrhosis or chronic viral hepatitis. In hepatitis or cirrhotic patients, any dominant solid nodule that is not clearly a hemangioma should be considered as HCC unless proven otherwise.39 Noninvasive criteria for diagnosing HCC suggested by the AASLD in 2005 consisted of serum AFP level >200 ng/mL or a typical enhancement pattern (arterial enhancement and portal or delayed washed out) on dynamic imaging of hepatic mass >2 cm in a cirrhotic liver.32 The AASLD further validated the diagnostic accuracy of a single dynamic technique showing intense arterial uptake followed by “washout’’ of contrast in the venous-delayed phases in patients of chronic hepatitis B or cirrhosis of any etiology.31 But histologic diagnosis of HCC is still recommended for patients who plan to have a nonsurgical therapy.40 For surgical patients, there is a concern over the possibility of tumor seeding from biopsy or fine-needle aspiration.40 The reported magnitude of the risk ranges from 1.6% to 5%.41–43 In patients who have coagulopathy or significant ascites, biopsy or fine-needle aspiration may be contraindicated. At the authors’ institution, fine-needle aspirations including cellblocks under ultrasound guidance are routinely performed.44 Core biopsy of the mass is reserved for patients in whom a definitive diagnosis cannot be made by fine-needle aspirations with cellblocks. Coaxial cannula is used with fine-needle or core biopsies to reduce the chance of needle track seeding.45 In patients who have small hepatic nodules with diagnostic possibilities ranging from well-differentiated malignancy to benign disease, ultrasonographically guided needle-core biopsy should be considered.46 Ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) are the most common modalities used to evaluate tumors in the liver. Ultrasound examination of the liver is commonly used as a screening tool; small tumors are often hypoechoic. As the tumor grows, the echo pattern tends to become isoechoic or hyperechoic, and HCC can be difficult to distinguish from the surrounding liver.47 Nodules <1 cm should be followed with ultrasonography again at intervals of 3 months. Nodules >1 cm in a cirrhotic liver should be investigated further with four-phase dynamic CT scan. If the four-phase dynamic CT scan cannot prove the diagnosis of HCC, contrast-enhanced MRI or biopsy is the next tool. The contrast-enhanced ultrasound was not recommended in the diagnosis of HCC because it may provide false-positive result in patients with cholangiocarcinoma.31 Extrahepatic metastases are not common at presentation; they occur mainly in patients with T4 disease. The most common sites of metastases are lung, abdominal lymph nodes, and bone.48 Routine metastatic surveys in patients with early-stage HCC are not recommended.
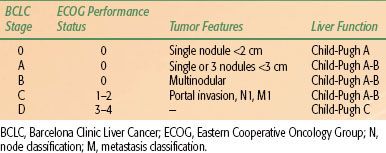
TABLE 60.1 BARCELONA CLINIC LIVER CANCER TAGING SYSTEM FOR HEPATOCELLULAR CARCINOMA
Staging
The prognosis in HCC patients is more complex because the underlying liver function also affects prognosis. Several factors have been identified as being important determinants of survival: the severity of underlying liver disease, the size and number of the tumor, vascular invasion, regional lymph node metastasis, and the presence of distant metastases. There is no worldwide consensus on the use of any given HCC staging system. However, most major trials of HCC therapy have chosen the Barcelona Clinic Liver Cancer Staging System (Table 60.1), making it the de facto reference staging system.31 Other systems, such as the American Joint Committee on Cancer’s TNM (tumor, node, metastasis) staging system,49 Okuda staging systems,50 and the Cancer of the Liver Italian Program scoring system,46 are also commonly used.
Pathologic Classification
Histologic classification of malignant tumors of the liver includes HCC (conventional), HCC (fibrolamellar variant), cholangiocarcinoma (intrahepatic bile duct carcinoma), mixed hepatocellular cholangiocarcinoma, undifferentiated carcinoma, and hepatoblastoma. The fibrolamellar variant of HCC has a relatively better prognosis. It occurs more frequently in adolescents or young adults and has a more indolent clinical course than conventional HCC.51 Hepatoblastoma occurs most commonly in young children (median age, 13 to 16 months) and usually presents in an advanced stage.52,53
General Management
HCC is often a multicentric disease, especially when it is associated with HCV. The incidence of multicentric disease in HCV-related HCC (53.3%) is significantly higher (P <.05) than in the non-HCV-related HCC (7.7%).54 The risk of multicentric occurrence increases with the progression of chronic liver disease and cirrhosis.55,56 Although multiple tumors occur less often in HBV-associated HCC than in HCV-associated HCC, the incidence of intrahepatic recurrence of HCC is significantly higher in patients with a sustained HBeAg-positive and high serum concentration of HBV DNA.57 Despite these observations, the mainstay of therapy is surgical resection. The majority of patients, however, are not eligible for surgery because of the extent of tumor involvement or underlying liver dysfunction. Several other treatment modalities are available, including liver transplantation, radiofrequency ablation (RFA), percutaneous ethanol or acetic acid ablation, transcatheter arterial chemoembolization (TACE), cryoablation, radiation therapy, and systemic chemotherapy.
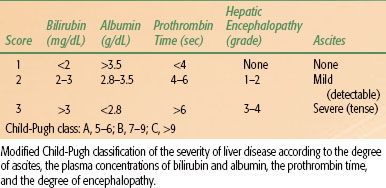
TABLE 60.2 GRADING SYSTEM FOR CIRRHOSIS: THE CHILD-PUGH SCORE
Surgical Resection
Surgical resection is a reliable method to obtain long-term disease control. The main limiting factor for resection is liver function. In virus-related HCC, the extent of surgical resection of hepatic tumor depends on the functional reserve of the residual liver after surgery. Previously, the selection of patients for resection has been based on the Child-Pugh classification (Table 60.2), but this method is known to be inconsistent. Many Japanese and other Asian investigators rely on the ICG retention test.57 In Europe and the United States, selection of optimal candidates for resection is usually based on the assessment of the presence of portal hypertension, which is assessed clinically, or by hepatic vein catheterization.58 Clinically significant portal hypertension is suspected when the platelet count is below 100,000/mm3, which is associated with significant splenomegaly. The goal of surgery is to remove gross tumor with a margin of 1 to 2 cm of normal liver. Today, however, the 5-year survival after resection can exceed 50%.
Liver Transplantation
Many patients are inappropriate for definitive surgery because of underlying liver dysfunction. The increasing availability of liver transplantation has made this procedure an alternative to tumor resection for selected patients. Based on the Milan study and others, liver transplantation is an effective option for HCC patients.59 The selection criteria are solitary tumor 5 cm or up to three nodules with tumor size <3 cm. When these selection criteria are strictly applied, excellent overall 3- to 4-year actuarial (75% to 85%) and recurrence-free survival rates (83% to 92%) can be achieved.59–61 Risk factors of recurrence after transplantation include tumor size, number of tumors, vascular invasion, and persistence of HBV infection.61–63 A major disadvantage with orthotopic liver transplantation is the unpredictable, potentially long waiting time for donor organs.63 Living donor transplantation can be offered for HCC if the waiting time would be so long that there is a high risk of tumor progression leading to exclusion from the waiting list.
Percutaneous Ablation
For patients with early-stage HCC who are not suitable for resection or transplantation, percutaneous ablation would be the treatment option.64,65 Destruction of tumor cells can be achieved by the injection of chemical substances (ethanol, acetic acid) or by modifying the temperature (radiofrequency, microwave, laser, cryotherapy). Ethanol injection is highly effective for small HCC. It achieves a necrosis rate of 90% to 100% of the HCC <2 cm, but the necrosis rate could be reduced to 50% in HCC >3 cm.66,67,68
RFA involves the local application of radiofrequency thermal energy to the lesion. RFA is a reasonable option for patients who do not meet resectability criteria for HCC yet are candidates for a liver-directed procedure based on the presence of liver-only disease. The best outcomes are in patients with a single tumor <4 cm in diameter. The efficacy in tumors >2 cm is better than with ethanol. Randomized control trials comparing RFA and ethanol injection have shown that RFA provides better local disease control that could result in an improved survival.69–72
Transcatheter Arterial Chemoembolization
HCC is a highly vascular tumor supplied mostly by the hepatic or adjoining arteries and has strong neoangiogenic activity during its progression. This character provides the pathologic basis for the radiological diagnosis of disease. Its also supports arterial obstruction as a treatment option. TACE combines selectively injecting chemotherapeutic agents through the tumor artery followed by intra-arterial embolization of tumor artery with lipiodol, an iodized oily contrast agent, despite two prospectively randomized studies that failed to show significant survival benefit over conservative management in patients with unresectable HCC.73,74 Systematic review of randomized prospective studies in more recent literature has shown TACE to have a positive impact on survival.75 For palliative purpose, TACE has been accepted as the standard treatment for patients with unresectable HCC, and it can be used selectively for tumors of different locations and can be repeated if necessary.1,76
Radiotherapy
HCC is a radiosensitive tumor.38 The major drawback of radiotherapy in treating HCC is the poor radiation tolerance of adjacent normal liver and the difficulty of tumor localization.77,78 Recent technological and conceptual developments in the field of radiation therapy, such as intensity-modulated radiation therapy, image-guided radiation therapy, respiratory gating, and stereotactic body radiation therapy, have the potential to improve radiation treatments by conforming the delivered radiation dose distribution tightly to the tumor or target volume outline while sparing normal liver tissue from high-dose radiation.79,80 For patients with liver-confined disease treated with conformal radiotherapy with or without TACE, local control response rates ranged from 40% to 90%, and the median survival ranged from 10 to 25 months.81 Indications for conformal radiotherapy include large unresectable HCC, relieving portal vein thrombosis and obstructive jaundice, failure of prior TACE and as part of combined modality treatment with TACE, and percutaneous ablation therapy.38,82,83 The combination of TACE and conformal radiotherapy in large unresectable HCC has not yet been defined in any randomized trial.
Radiation Doses
The tumor response to radiotherapy and survival of patients with HCC are related to the dose delivered.82,84 Partial response was 18% to 23% in unresectable HCC treated with 21 Gy in 7 fractions and increased to 48% with dose around 33 Gy.85,86 Seong et al.87 irradiated 27 HCC patients who failed TACE and observed an objective response rate of 67% after a mean dose of 51.8 Gy ± 7.9 Gy. Dawson et al.84 escalated radiation doses for unresectable hepatobiliary cancer and observed that patients who received radiation doses >70 Gy had better median survival (>16.4 months). It appears that the higher the radiation doses given, the higher the tumor response seen (Table 60.3).
Caution should be given in HBV-related HCC; local radiotherapy may deteriorate liver function by HBV activation, so prophylactic anti-HBV treatment should be considered.
TABLE 60.3 RADIOTHERAPY WITH AND WITHOUT CHEMOTHERAPY FOR UNRESECTABLE HEPATOCELLULAR CARCINOMA
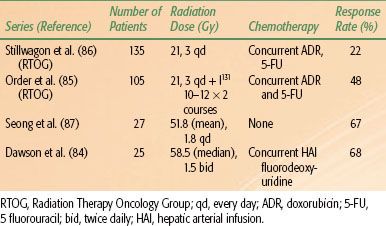
TABLE 60.4 RADIATION TREATMENT GUIDELINES FOR HEPATOCELLULAR CARCINOMA
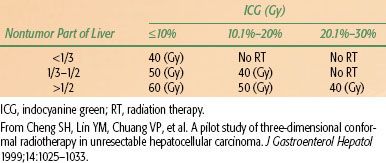
TABLE 60.5 COMBINATION TRANSCATHETER ARTERIAL CHEMOEMBOLIZATION AND LOCAL RADIOTHERAPY IN UNRESECTABLE HEPATOCELLULAR CARCINOMA
Treatment Volumes
Although higher radiation doses have been shown to produce a higher response, patients with HCC usually have liver cirrhosis, which will not allow such doses. Published reports on three-dimensional conformal radiation therapy (3D-CRT) with photon energy suggest that portions of the liver can be treated with higher doses and acceptable complications. McGinn et al.88 developed a normal tissue complication probability (NTCP) for intrahepatic malignancy. They designed a protocol in which each patient received the maximum possible dose while being subjected to a 10% risk of radiation-induced liver disease based on the NTCP model. The mean doses delivered according to this protocol were 56.6 ± 2.31 Gy (range, 40.5 to 81 Gy). They observed a complication rate of 4.8% (95% confidence interval, 0% to 23.8%), a number that did not differ significantly from the predicted 8.8% calculated on the basis of the NTCP model. This model, however, was derived using patients with relatively normal liver function, and only 4 of 21 of their study population had HCC. The NTCP model for patients with impaired liver function requires further evaluation.89
Multivariate analyses demonstrated that the severity of hepatic cirrhosis was the only independent predictor for radiation-induced liver disease. For cirrhotic patients with Child-Pugh grade A, the hepatic radiation tolerance was a mean dose to normal liver of 23 Gy.90 These authors also suggested a dose–volume histogram for irradiation to normal liver so that the irradiated hepatic volume over 10 Gy, 20 Gy, 30 Gy, and 40 Gy should be less than 68%, 49%, 28%, and 20%, respectively.
ICG retention can be used to guide the treatment of HCC. In HCC patients with normal bilirubin and without ascites, if the ICG retention 15 minutes (ICG 15) is normal, the resection volume can be trisegmentectomy or bisegmentectomy; if ICG 15 is 10% to 19%, the resection volume can be left lobectomy or right monosegmentectomy; if ICG 15 is 20% to 29%, the resection can be subsegmentectomy; if ICG 15 is 30% to 39%, the resection can be done to only a limited area of the liver.91 Therefore, the authors propose a treatment guideline using the ICG test for 3D-CRT for patients with impaired liver function (Tables 60.4 and 60.5). The authors advise no radiation treatment for patients with Child-Pugh class C liver cirrhosis or prolonged ICG retention unless only a very small portion of the liver is included in the radiation treatment fields.38
Stereotactic body radiotherapy (SBRT) to the intrahepatic lesion becomes the option of treatment with the improvement of planning system and linear accelerators. But the large fraction size makes a difference in normal tissue tolerance. There have been many retrospective studies but no universal consensus for hepatic tolerance of SBRT. Son et al.96 reported that at least 800 mL of normal liver had to receive a total dose <18 Gy to reduce the risk of the deterioration of hepatic function. For lesions beside the duodenum or stomach, Mahadevan et al.97 reported that 24 to 36 Gy (depending on the distance from the duodenum and stomach) in 3 fractions had acceptable side effects.
Design of Treatment Field
The goal of conformal radiotherapy is to precisely target the tumor(s) and to reduce damage to the surrounding normal tissue. Respiratory organ motion probably is the largest intrafractional organ motion. Aruga et al.98 studied organ motion involving the use of CT images obtained during both the static exhalation phase and static inhalation phase for upper abdomen irradiation. They found that the tumor shifted between the two respiratory phases. The variation ranged from 2.6 to 23.7 mm: from 0.4 to 5.9 mm in the lateral direction, 2.2 to 24.5 mm in the longitudinal direction, and 0.2 to 11.7 mm in the vertical direction. Breath-gating or breath-holding irradiation may help overcome the problem of respiratory movement during irradiation.99 In general, the radiation-field margin to the target in the lateral direction should be 6 to 9 mm, vertical direction 9 to 12 mm, superior direction 10 mm, and inferior direction 19 to 21 mm.100,101 Controlling, gating, or tracking respiratory motion or by the use of image-guided radiation therapy, as previously mentioned, is currently under investigation.102
Acute and Late Complications
The dose-limiting tissue injuries in radiation treatment for HCC include the liver, stomach, duodenum, bowels, and kidneys. Acute complications include general fatigue, transient elevation of liver function test, nausea and vomiting (mainly for tumors in the left lobe of the liver), fever, and pancytopenia.38,87 Subacute and late complications include hepatic failure, radiation pneumonitis, and gastrointestinal bleeding (especially in tumor located in the inferior portion of the right lobe of the liver and radiation doses >50 Gy).38,103 Hepatic failure can be avoided by an appropriate selection of patients and careful treatment planning.
Results of Combining Transcatheter Arterial Chemoembolization and Local Radiation Therapy
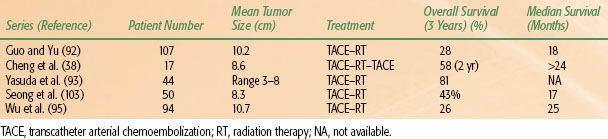
TACE alone rarely produces complete pathologic remission for HCC >5 cm, especially in the peripheral zone of the tumor.104,105 Additional therapy theoretically is required to eradicate the residual disease. The combination of TACE and conformal radiotherapy shows promising results in large HCC (see Table 60.5). Guo and Yu92 reported 107 patients with large unresectable HCC treated with TACE followed by external-beam irradiation. The greatest dimension of the tumors ranged from 5 to 18 cm. After a median follow-up interval of 24 months, the cumulative survival rates at 3 and 5 years were 28.4% and 15.8%, respectively, with a median survival of 18 months. Cheng et al.38 reported on 17 patients with unresectable HCC treated with TACE and conformal radiotherapy. The mean tumor size was 8.6 cm (range, 3.7 to 18 cm). The overall survival rate at 2 years was 58% and local progression-free tumor control was 83%. After 24 months of median follow-up, the median survival had not been reached, and 4 of 17 patients remained progression free (Fig. 60.2).
Another study that combined TACE and local radiotherapy in 50 unresectable HCC patients reported a partial response rate of 66% and survival rates at 3 years of 43%.103 Wu et al.95 reported 94 patients with HCC received 3D-CRT combined with TACE. The response rate was 90.5%. The overall survival rates at 1, 2, and 3 years were 93.6%, 53.8%, and 26.0%, respectively, with the median survival of 25 months.
Patients with branch portal vein thrombosis may benefit from combined treatment. Tazawa et al.106 reported on 19 patients with thrombus in the first branch of portal vein who received 3D-CRT with TACE. Eleven patients (58%) had an objective response, and the 1-year survival rate was 41% (eight patients). Although we do not know yet whether combined TACE and conformal radiotherapy is a superior treatment modality to TACE alone, the results of these studies suggest that in patients with large unresectable HCC, combined treatments could be a promising new treatment modality worthy of further investigation.
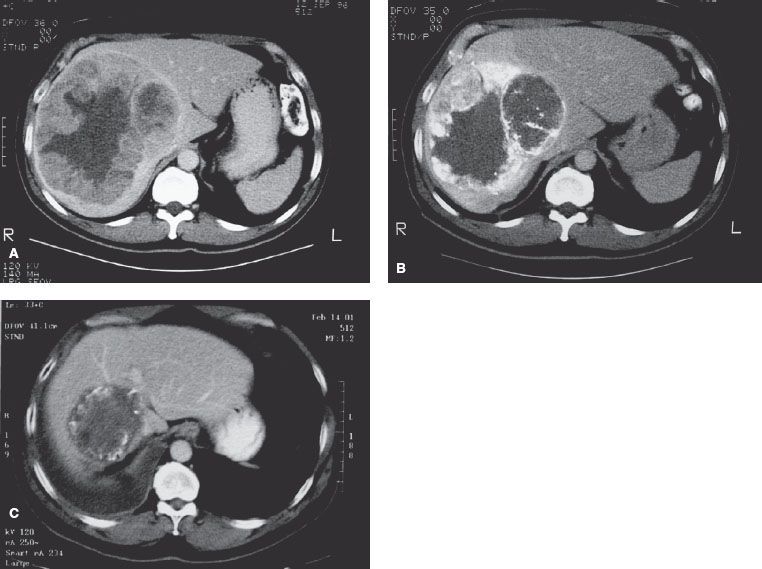
FIGURE 60.2. A: A 55-year-old man with hepatitis B virus infection developed a large hepatocellular carcinoma in right lobe of liver. The tumor measured 12 é 15 é 15 cm. Alfa-fetoprotein (AFP) was 901 ng/mL. B: After a first course of transcatheter arterial chemoembolization (TACE), his AFP dropped to 150 ng/mL. It rose to 440 ng/mL 3 months later. He had second TACE followed by three-dimensional conformal radiotherapy. This image was taken after the second TACE, revealing viable tumors in the peripheral zone of the main mass. C: Four years and 4 months after the first treatment, computed tomography revealed a nonenhancing tumor in the right lobe of the liver. His AFP remained <10 ng/mL after completion of radiotherapy.
Chemotherapy
Systemic chemotherapy for HCC has limited value in clinical practice because only a small portion of patients obtain significant benefits at the price of remarkable toxicity.107 In general, cytotoxic therapy should be reserved for medically appropriate patients with adequate hepatic function, preferably administered within the context of a clinical trial. The side-effect profile of any chemotherapy regimen should be considered carefully in patients with advanced liver disease and a short life-expectancy.
Several new drugs have been tested recently. One of the more promising of these drugs is gemcitabine. It has a low toxicity profile, but its antitumor activity in patients with advanced HCC is marginal: the response rate is 18% and the response duration is 35 weeks.108 Combination chemotherapy regimens have shown better responses. Leung et al.109 reported on 50 patients treated with intravenous chemotherapy composed of cisplatin, doxorubicin, 5-fluorouracil (5-FU), and interferon-alfa. The partial response was 26% (13/50) and four patients achieved pathologic complete remission. Louafi et al.110 reported 34 patients of advanced HCC treated with gemcitabine and oxaliplatin. The disease control rate was 76%. Median progression-free and overall survival times were 6.3 months and 11.5 months, respectively.
Molecular Targeted Therapy
Recent advances in molecular biology have uncovered the structures and functions of many cytokines thought to have a strong relationship with the mechanisms of the antitumor effect of biological therapies. Thalidomide, a sedative previously associated with severe fetal malformations, has limited value in the treatment of HCC.111,112 Molecular targeted therapy to the epidermal growth factor receptor and vascular endothelial growth factor has exhibited the potential to inhibit tumor growth of HCC.113 The randomized trial of sorafenib has showed statistic significance in median overall survival (10.7 vs. 7.9 months) and median time to radiological progression (5.5 vs. 2.8 months).114
Areas of Failure and Cause of Death
Survival of HCC after treatment depends on the clinical stage and liver function. Five-year survival in resectable HCC after partial hepatectomy is 50% to 73% in stage I patients, 30% to 56% in stage II patients, 10% to 29% in stage III patients, and ~10% in stage IV patients.115,116 The 5-year survival for patients with unresectable HCC is usually <10%.117 The major cause of failure after resection is tumor recurrence within the liver; this observation still holds true for patients who undergo TACE alone. Extrahepatic metastases in advanced-stage HCC have become more common in recent years, related to improvements in the local treatment of intrahepatic disease.118,119
Future Study
The overall treatment outcome in HCC is generally unsatisfactory. The main cause of failure is intrahepatic recurrence. Future efforts should be directed toward prevention of HBV and HCV infection through vaccination in hyperendemic regions and perhaps through chemoprevention for individuals who are already infected and are persistent carriers. Other approaches include early diagnosis in high-risk populations and development of effective adjuvant treatments after resection.
 BILIARY TRACT CANCER
BILIARY TRACT CANCER
Biliary tract cancers consist of cancer of the gallbladder, the bile ducts, and the ampulla of Vater. They are highly lethal because most are locally advanced at diagnosis. Gallbladder cancer is the most common cancer of the biliary tract and accounts for two-thirds of these cancer patients, whereas bile duct cancer accounts for the remaining one-third.120 The term cholangiocarcinoma was used to describe cancers arising from the epithelial cells of the bile ducts, which include intrahepatic, perihilar, and distal extrahepatic biliary tree. At present, surgical excision of all detectable biliary tract cancers is associated with improvement in long-term survival. For unresectable tumors, the purpose of treatment is to palliate symptoms such as obstructive jaundice, biliary tract infection, pain, and ascites.
 CHOLANGIOCARCINOMA
CHOLANGIOCARCINOMA
Topographic Anatomy
The bile ducts originate within the liver, with the left and right hepatic ducts joining to form the common hepatic duct. At the origin of the cystic duct, it becomes the common bile duct. The cystic duct drains bile from the gallbladder into the common bile duct. The gallbladder is adjacent to the undersurface of the liver.
There is a rich lymphatic network along the submucosa of bile ducts. The primary lymphatic drainage of the biliary tract is to the lymph nodes in the pericholedochal area, periportal region, hepatoduodenal ligament, common hepatic artery, and pancreaticoduodenal groups.80,121,122
Epidemiology and Risk Factors
Cholangiocarcinoma is a rare tumor in developed countries; there are approximately 2,000 to 3,000 cases per year in the United States. However, it is one of the most common cancers in endemic areas of developing countries, as high as 87 per 100,000 people in northeast Thailand.123 Cholangiocarcinoma accounts for about 15% of the primary liver cancer worldwide. But the proportion was different in different regions: 20% in Western countries, <10% in Asian nations, and as high as 90% in north Thailand.64,124
A number of risk factors have been identified as important in the development of cholangiocarcinoma, most of which share a history of long-standing inflammation and chronic injury of the biliary epithelium.125 The major risk factor in Western countries is primary sclerosing cholangitis, which is closely associated with chronic inflammatory bowel disease, particularly ulcerative colitis.126 The risk of developing cholangiocarcinoma is higher in patients with primary sclerosing cholangitis, ulcerative colitis, and colonic neoplasm than in patients with primary sclerosing cholangitis and ulcerative colitis without colonic neoplasm.127 Studies in Japan and the United States also showed that chronic hepatitis C infection elevated the incidence of intrahepatic cholangiocarcinoma, with the odds ratio between 4 and 17.128,129 In Asia, chronic infections of the biliary tract and infestation by certain liver flukes, such as Clonorchis sinensis and Opisthorchis viverrini, are associated with cholangiocarcinoma and hepatolithiasis.123 Hepatolithiasis itself is also a risk factor for cholangiocarcinoma; 5% to 10% of patients with intrahepatic stones develop this complication.130,131 Moreover, the combination of liver fluke infestation and nitrosamine exposure may explain the very high incidence of cholangiocarcinoma in northeast Thailand.132 Other risk factors, although rare, include congenital fibropolycystic disease of the biliary system such as choledochal cysts and Caroli disease (cystic dilatation of intrahepatic bile ducts).133 The observed incidence and mortality of intrahepatic cholangiocarcinoma has increased in the past 3 decades in Japan, the United States, and the United Kingdom.14,134,135 In the United States, the age-adjusted mortality rate increased from 0.07 per 100,000 in 1973 to 0.69 per 100,000 in 1997; the estimated annual percentage change of mortality was 9.44%. Better case ascertainment and diagnosis because of improved diagnostic imaging, use of image-guided biopsies, or increased use of endoscopic retrograde cholangiopancreatography (ERCP) cannot fully explain this observation.135,136
Diagnosis
The most common presenting symptoms of biliary tract cancer are caused by obstruction of the bile duct. Those include painless jaundice, clay-colored stool, tea-colored urine, and pruritus. Other signs and symptoms include abdominal pain, fever, general malaise, abdominal distention, fullness, anorexia, and weight loss. The spectrum of cholangiocarcinoma can be classified into three broad groups: (a) intrahepatic, (b) perihilar, and (c) distal tumors. The age of onset is similar among the three groups and ranges from 60 to 65 years.137 Patients with extrahepatic tumor usually present with jaundice and tea-colored urine. Patients with intrahepatic tumors are less likely to be jaundiced and more likely to present with abdominal symptoms.
There are no reliable screening methods; early diagnosis is almost impossible even in patients with high-risk situations such as primary sclerosing cholangitis and hepatolithiasis.138 Some patients are diagnosed when screening blood work demonstrates elevation of alkaline phosphatase and γ-glutamyl transferase. Ultrasonography and CT are the initial primary tools to evaluate biliary tract tumor (Table 60.6). Further tests include percutaneous transhepatic cholangiography, ERCP with brushing cytology, serum carbohydrate antigen-19-9 (CA-19-9) levels, radiologic imaging with dynamic CT scan, MRI, or both, and angiography.
A serum CA-19-9 value >100 U/mL has a sensitivity of ~75% and a specificity of ~80%.139 The optimal cutoff value for serum CA-19-9 that best discriminates between benign or malignant biliary tract diseases is influenced by the presence of cholangitis. Thus, in patients with symptoms of acute cholangitis, serum CA-19-9 concentrations should ideally be re-evaluated after recovery. The sensitivity of serum carcinoembryonic antigen (CEA) is low and helpful in only one-third of patients.140 Biliary CEA levels increase significantly in patients with cholangiocarcinoma and also in patients with intrahepatic cholelithiasis (average, 50.2 to 57.4 ng/mL) compared with patients with benign strictures (average, 10.1 ng/mL) and patients with sclerosing cholangitis and choledochal cysts (average, 20.0 to 21.6 ng/mL).139 Serum AFP may increase in some cases of cholangiocarcinoma, and this would suggest a diagnosis of mixed HCC and cholangiocarcinoma.141
Pathology
Cholangiocarcinomas arise from the epithelium of the biliary tract; the majority of these cancers (>90%) are adenocarcinomas. Squamous cell carcinoma is the second most histologic type. Rare histologies include mucoepidermoid carcinoma, cystadenocarcinoma, and carcinoid tumor.
Adenocarcinomas are divided into three type: sclerosing, nodular, and papillary. Sclerosing tumors are characterized by an intense desmoplastic reaction. This type of tumor tends to invade the bile duct wall early and, as a result, is associated with low resectability and cure rates. Most cholangiocarcinomas are of this type.137 The nodular cholangiocarcinomas are also highly invasive tumors. Most patients have advanced disease at diagnosis. The resection and cure rates are both very low. In contrast, papillary type tumors usually present as masses in the lumen of bile duct, causing biliary obstruction early in the course of disease. Therefore, these tumors have the most favorable prognosis.142 Microscopically, cholangiocarcinoma are classically well differentiated to undifferentiated. Cells tend to be cuboidal or low columnar and resemble biliary epithelium; mucin is always demonstrable in the cytoplasm. Bile duct obstruction can be associated with reactive hyperplasia of subepithelial mucous glands with or without cholangiocarcinoma.143 Cholangiocarcinomas frequently invade lymphatic, perineural, and periductal spaces and the portal tracts. Spread along the lumen of the large bile ducts can also be seen, especially in perihilar cholangiocarcinoma.144
The differential diagnosis of cholangiocarcinoma from HCC can be further affirmed by positive reaction with CEA, CA-19-9, and immunohistochemistry with cytokeratin-19.145 Mutations in the p53 tumor suppressor gene and K-ras proto-oncogene have been identified in cholangiocarcinoma.146–148 p53 overexpression and K-ras mutations are associated with a shortened survival.146
TABLE 60.6 DIAGNOSTIC WORKUP FOR CARCINOMA OF THE BILE DUCT





