Cancer of the Colon and Rectum
 ANATOMY
ANATOMY
The colorectum consists of the cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, and rectum. Variability in the peritoneal investment, bowel mobility, and lymph node drainage of the colon and rectum presents unique therapeutic issues.1
The posterior and lateral surfaces of the ascending and descending colon are in direct contact with the retroperitoneum, whereas the anterior surface is draped with peritoneum.1 These posterior attachments can prevent significant mobility, increasing the difficulty of surgical resection. In contrast, the transverse colon is completely surrounded with peritoneum and supported on a long mesentery. As the sigmoid colon evolves distally into the rectum, the peritoneal coverage recedes. The rectum, approximately 12 to 15 cm in length, extends from the rectosigmoid junction to the puborectalis ring. The upper one-third of the rectum is draped with peritoneum anteriorly and on both sides. As the middle one-third of the rectum moves deeper into the pelvis, only the anterior surface is covered with peritoneum, which forms the posterior border of the rectouterine pouch or rectovesical space. The lowest one-third of the rectum is devoid of peritoneal covering and in close proximity to adjacent structures, including the bony pelvis (Fig. 61.1). Distal rectal tumors have no serosal barrier to invasion of adjacent structures and are more difficult to resect, given the close confines of the deep pelvis.
Colonic nodal drainage consists of pericolic nodes and nodes in association with the vascular supply to the colon (i.e., mesenteric nodes). Because of the mobile and extensive nature of the colonic mesentery, complete regional lymph node coverage with external-beam radiotherapy (EBRT) is challenging but is usually well treated surgically. In contrast, the major regional groups for rectal nodal drainage can be covered within a reasonable EBRT field and include the perirectal, presacral, and internal iliac nodes.
 EPIDEMIOLOGY AND RISK FACTORS
EPIDEMIOLOGY AND RISK FACTORS
Colorectal cancer (CRC) remains a major worldwide health problem. In the United States alone, it was estimated that there would be 143,460 patients diagnosed with CRC and 51,690 deaths in 2012.2 Worldwide, approximately 1.2 million new cases per year are diagnosed, with 600,000 deaths.3 In the United States, incidence of CRC over the last two decades declined by 2% to 3% annually; this is largely attributed to improvements in cancer prevention, early detection, and treatment.4
Genetic and environmental factors can increase the likelihood of developing CRC. A number of hereditary CRC syndromes exist, including familial adenomatous polyposis (FAP), MUTYH-associated polyposis, and Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]). Factors shown to increase the risk of developing CRC include the following: increasing age; male sex; family history of colorectal cancer; inflammatory bowel disease; increasing height; increasing body mass index; consumption of processed meat, refined grains, starches, and sugars; excessive alcohol intake and smoking; and low folate consumption.5,6 Of these risk factors, only increasing age, male sex, and excessive alcohol use have been associated with rectal cancer.7 Age is a major risk factor for the development of CRC, with median age of diagnosis in the seventh decade. Incidence rates increase dramatically between ages 40 and 50 years and each subsequent decade thereafter (data from Surveillance, Epidemiology, and End Results Program accessible online at www.seer.cancer.gov).
Although CRC may be linked to chemical carcinogens within the bowel lumen, it is not established whether these are ingested, the result of chemical activation of substances in the fecal stream, or a bacterial by-product.8,9 The value of consumption of fruits and vegetables in the prevention of CRC remains controversial, although recent studies suggested that these associations might have been overstated.10 Contemporary prospective and randomized data do not support a high-fiber diet in the prevention of CRC.11 Other studies suggested that nonsteroidal anti-inflammatory drugs may serve in reducing recurrent adenomas and CRC, but long-term therapy must be weighed against potential side effects. The role of chemopreventive agents (carotenoids, aspirin, and other nonsteroidal anti-inflammatory drugs) in colorectal cancer remains an area of active investigation. A detailed discussion of the biologic and genetic pathways of development of colorectal cancer is beyond the scope of this chapter. In brief, it has been established that the development of colon cancer is a multifactorial process, involving genomic instability, mutational inactivation of tumor suppressor genes, and activation of oncogene pathways. Microsatellites are mutated short-repeat DNA sequences, usually consisting of one to five nucleotides. The majority of patients with HNPCC, as well as a minority of sporadic colorectal cancers, harbor microsatellite instability. It has been shown that this instability occurs in patients with mutations in genes encoding enzymes that repair DNA replication errors. These defects in mismatch repair lead to high-frequency microsatellite and hence genomic instability.12 Studies suggested that patients with tumors possessing a high frequency of microsatellite instability have more-favorable outcomes and lower likelihood of developing metastatic disease.13 CRC appears to arise through inactivation of the tumor suppressor genes adenomatous polyposis coli (APC), P53, and TGF-β, as well as activation of the RAS, BRAF, and PI3 K proto-oncogenes.12 Further elucidation of the genetic pathways in the development of CRC remains an active area of investigation and may ultimately affect therapy of this disease.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
CRC often produces minimal or no symptoms, emphasizing the need for screening programs in the general population. Patients with symptomatic CRC most commonly experience abdominal pain, change in bowel habits, hematochezia/melena, weakness, iron-deficiency anemia, and weight loss.14,15 Less commonly, patients present with nausea, vomiting, or abdominal distention, which may be signs of tumor-related obstruction.
The clinical presentation of CRC is determined largely by site of the tumor. Cancers of the right colon are often exophytic and commonly associated with iron-deficiency anemia due to occult blood loss, resulting in delayed diagnosis. During the last 20 years, the incidence of cancer of the right colon appears to have increased and accounts for one-third of large-bowel cancers.16 Cancers of the left colon and sigmoid colon are often deeply invasive, annular (“apple core lesions”), and accompanied by obstruction, rectal bleeding, and alteration in bowel habits.
FIGURE 61.1. Idealized depiction of peritoneal relationships in the colon and rectum. The transverse and sigmoid colon are intraperitoneal, with a complete peritoneal covering (serosa) and mesentery. The ascending and descending colon are retroperitoneal, lack a true mesentery, and usually do not have a peritoneal covering posteriorly or laterally. The upper rectum begins above the peritoneal reflection and has peritoneum anteriorly and laterally. The lower one-half to two-thirds of the rectum is below the peritoneal reflection (infraperitoneal). (From Gunderson LL, O’Connell MJ. The postoperative chemotherapy/irradiation adjuvant strategy. In: Cohen AF, Winawer SJ, Friedman MA, et al., eds. Cancer of the colon, rectum, and anus. New York: McGraw-Hill, 1995;631–645; with permission.)
 SCREENING
SCREENING
Neoplastic polyps, including tubular adenomas, villous adenomas, and tubulovillous adenomas, are precursors of colon cancers.8,17 Most CRC arise from pre-existing polyps. As the cumulative lifetime risk of developing CRC in the United States is about 6%, screening programs for the general population have been initiated. The goal of screening is to detect preinvasive polyps or early invasive cancer. Mounting evidence supports that screening of asymptomatic, average-risk individuals can detect CRC at early, curable stage, thereby reducing CRC mortality.18,19–20
Given the data in favor of screening, health organizations such as the American Cancer Society (ACS) and the United States Preventative Services Task Force recommend screening in average-risk individuals starting at age 50. The ACS advocates for tests that detect adenomatous polyps and cancer as follows:
1. Flexible sigmoidoscopy every 5 years.
2. Double contrast barium enema every 5 years.
3. Computed tomography (CT) colonography every 5 years.
4. Colonoscopy every 10 years.19
5. Guaiac-based fecal occult blood, fecal immunohistochemical, and stool DNA tests may be performed for CRC, but not polyp, detection.21
In high-risk patients (patients with adenomatous polyps, history of CRC, first-degree relative diagnosed with CRC or adenomas, inflammatory bowel disease, or high risk due to family history or genetic testing), more-intensive surveillance is recommended. Individuals with FAP initiate annual sigmoidoscopy or colonoscopy beginning at age 10 to 12 years until age 35 to 40 years if negative. Patients with HNPCC initiate annual screening at age 20 to 25 years or 10 years prior to earliest familial CRC diagnosis.22,23 Patients with inflammatory bowel disease should initiate screening with colonoscopy 8 to 10 years after initial diagnosis.21 The American College of Gastroenterology recommends the following for high-risk individuals based on family history24:
1. Colonoscopy screening.
2. If single, first-degree relative diagnosed with CRC, or advanced adenoma at age 60+ years, screening every 10 years beginning at age 50 years.
3. If single, first-degree relative diagnosed with CRC, or advanced adenoma at age <60 years or two or more first-degree relatives with CRC or advanced adenoma at any age, screening beginning at age 40 years or 10 years before the youngest relative’s diagnosis. Screening should be performed every 5 years.
Although screening methods can detect colorectal cancer at an early stage, <40% of patients are diagnosed with early disease, likely reflecting low rates of disease awareness, as well as the infrequency of screening in eligible candidates.25 Approximately 50% to 60% of adults aged 50 to 75 years underwent CRC screening in 2008.26,27 Lower rates of screening are seen in younger patients, Hispanics, individuals of lower socioeconomic status, and individuals without insurance.27 Although all screening tests have potential drawbacks, patients should be educated regarding relative risks and benefits of screening modalities, including potential benefits in reducing risk of CRC.
 PATHOLOGY AND PATHWAYS OF SPREAD
PATHOLOGY AND PATHWAYS OF SPREAD
Tumors of the colorectum arise in the mucosa and virtually all (>90%) are adenocarcinomas.8 Other histologic types include squamous cell carcinoma, melanoma, small-cell carcinoma, carcinoid, sarcoma, and lymphoma. Most grading systems classify adenocarcinoma as well, moderately or poorly differentiated. Large-bowel tumors invade from mucosa through the bowel wall and beyond, with involvement of lymphatic channels and lymph nodes. Hematogenous spread can occur, primarily to the lung and liver. There is little propensity for colon cancer to spread longitudinally within the bowel wall, in contrast to esophageal or gastric cancers.
 PATIENT EVALUATION/STAGING
PATIENT EVALUATION/STAGING
Workup should include a complete history and physical exam, including digital rectal examination (DRE). On DRE, size, location, distance from the verge, mobile versus fixed, and sphincter function should be noted. Pelvic exam should be performed in women diagnosed with rectal cancer to assess for vaginal involvement where appropriate. Pretreatment evaluation should include pathologic confirmation of adenocarcinoma, colonoscopy to evaluate extent of tumor and rule out synchronous primaries (occurring in 1% to 5%), and baseline lab tests, including blood counts, liver function tests, and carcinoembryonic antigen levels.9 India ink may be used at the time of colonoscopy to mark the proximal and distal disease extent.
Patients with CRC should undergo abdominal/pelvic CT scan and chest x-ray or chest CT to evaluate extent of local-regional disease, as well as the presence or absence of distant metastases. Efforts to improve the clinical assessment of rectal cancers have been enhanced considerably with the evolution of new imaging modalities. CT appears to be more useful in identifying enlarged pelvic lymph nodes and metastasis outside the pelvis than the extent or stage of the primary tumor.28 Standard CT does not permit the visualization of the layers of the rectal wall, and therefore its utility in the assessment of smaller primary cancers is limited.29 The sensitivity of CT scan is reported as 50% to 80% accurate, with a 30% to 80% specificity (65% to 75% accurate for tumor staging and 55% to 65% accurate in mesorectal lymph node staging).30 The ability of CT scans for detecting distant metastasis, including pelvic and para-aortic lymph nodes, is higher than for detecting perirectal nodal involvement (75% to 87% vs. 45%).31,32 Any lymphadenopathy near the rectum seen on a CT scan should be considered abnormal.
For rectal malignancies, endoscopic ultrasound (EUS) or pelvic magnetic resonance imaging (MRI) can assess primary disease and, with less sensitivity, evaluate nodal extent. Transrectal EUS techniques have been more helpful in efforts to clinically stage rectal cancers. EUS is 80% to 95% accurate in tumor staging and 70% to 75% accurate in mesorectal lymph node staging.33,34 Transrectal ultrasound is able to visualize layers of the rectal wall, including the mucosa, muscularis mucosa, submucosa, and muscularis propria.35,36 Its use is more limited in tumors of the upper rectum or for stenosing tumors. EUS can also identify enlarged perirectal lymph nodes but is not effective outside of the perirectum.37 One situation in which EUS can be very useful is in determining extension of disease into the anal canal, which is an area that is poorly visualized on CT but of critical importance for planning sphincter-preserving surgical procedures.38
More recently, MRI techniques have been found to be of high accuracy in defining the extent of rectal cancer extension into the mesorectum and in determining the location and stage of tumor.39,40 Different approaches to MRI have been explored, including the use of body coils, endorectal MRI, and phased-array techniques. Although MRI appears to have high levels of accuracy, it requires a significant learning curve but is becoming a greater part of the standard presurgical workup for rectal cancer. Body-coil MRI, which first became available in the mid 1980s, has had an accuracy of 54% to 66% for T staging, but this has improved with the use of endorectal coil MRI, with reported accuracy rates of 80% to 95%.16,41 A significant advantage of both endorectal and surface coil MRI is that it is less operator dependent and permits a larger field of view than EUS. It also allows assessment for proximal tumors and stenotic lesions where EUS is not possible. Another advantage of MRI is that it can detect involved lymph nodes on the basis of characteristics other than size. MRI can also be helpful in determining the extent of lateral extension of disease, which is critical in predicting the adequacy of circumferential margins for surgical excision.17 Several studies using phased-array MRI reported accuracy rates of 80% to 97% in predicting lateral disease extent and correlated the likelihood of tumor-free resection margin by visualizing tumor involvement of the mesorectal fascia.42
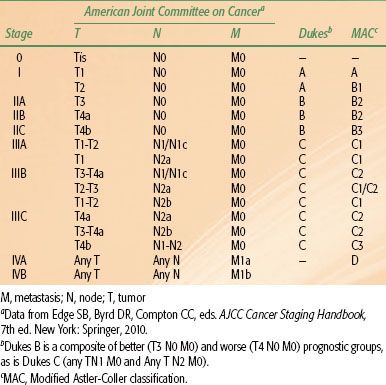
Positron emission tomography (PET) scan, although less accurate in assessment of primary disease, is useful in evaluating patients with oligometastatic disease who may be appropriate candidates for resection of metastatic sites with curative intent.43 Liver MRI is considered the test of choice in assessment of hepatic metastases in patients with CRC.44 All of these imaging techniques have advantages and limitations and should be considered complementary to physical examination. They are all less accurate in predicting response after neoadjuvant therapy, with high rates of false positivity, and should be interpreted with caution in this setting.45,46 Prognostic factors influencing survival in CRC patients include depth of tumor invasion into and beyond the bowel wall, the number of involved regional lymph nodes, and the presence or absence of distant metastases. The tumor, node, metastasis (TNM) system of the American Joint Committee on Cancer can be used as a clinical (preoperative) or postoperative staging system (Tables 61.1 and 61.2).
 TREATMENT OF COLON CANCER
TREATMENT OF COLON CANCER
Surgery
Surgery is the primary treatment modality for patients with colonic tumors. Resection with curative intent is possible in approximately 75% of patients.9 Surgery of primary colon cancer is based on the anatomy and mechanisms by which this disease spreads. Adenocarcinomas of the colon may grow by direct extension into the lymphatics of the submucosa and bowel wall. To avoid cutting across tumor intramural lymphatics, sufficient lengths of bowel must be resected proximal and distal to the primary cancer. Colon cancer often extends through the serosa into mesenteric lymphatics that run along the blood vessels draining into the portal watershed at the root of the mesentery. Resection includes removal of the major lymphatic drainage system in the mesentery. Because anatomic resections are designed to include named blood vessels and draining lymphatics, the boundaries for resecting large-bowel cancer are relatively uniform. Right hemicolectomy, transverse colectomy, left hemicolectomy, and sigmoid resection are performed by adherence to surgical oncologic principles without major sacrifice of large-bowel function. Consensus guidelines recommend that a minimum of 12 lymph nodes should be excised for appropriate staging.47,48–49 As with other gastrointestinal and pelvic malignancies, the use of laparoscopic techniques has increased. Data suggest no difference in recurrence or survival outcomes with open versus laparoscopic resection.50–53
Resection results in excellent cure rates for lesions limited to the bowel wall with negative nodes (average 5-year survival, 97% for T1 N0; 85% to 90% for T2 N0). With a single high-risk feature of extension beyond the colonic wall (T3–4 N0) or involved nodes (T0–2 N+), 5-year survival with surgery falls to 65% to 75%, and adjuvant treatment is often indicated. When both high-risk features are present (T3–4 N+), 5-year survival with surgery alone drops to approximately 50% (T3 N+) and 35% (T4 N+), and adjuvant treatment is recommended.
TABLE 61.1 AMERICAN JOINT COMMITTEE ON CANCER 2010 TNM STAGING OF COLORECTAL CANCER
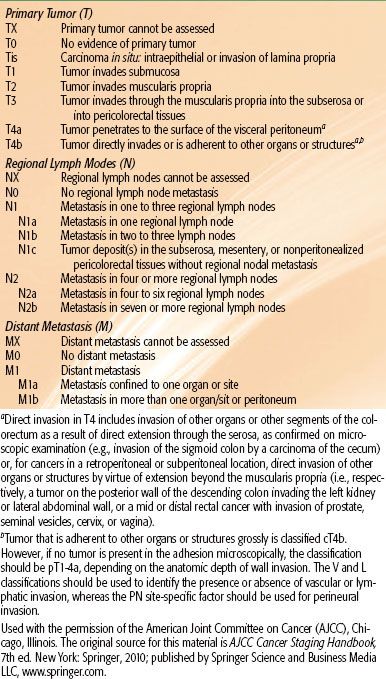
TABLE 61.2 STAGING OF COLON AND RECTUM CANCER
Adjuvant Chemotherapy
The benefit of adjuvant chemotherapy has been clearly demonstrated in stage III patients, whereas benefit in stage II patients is more controversial. Prospective randomized trials have shown that the addition of 5-flourouracil (5-FU) and leucovorin (LV) improves survival for resected stage III patients.54,55 More recently, newer agents have been investigated and have shown potential benefit. Capecitabine, an oral 5-FU prodrug, demonstrated similar overall survival (OS) and disease-free survival (DFS) rates to 5-FU/LV in patients with resected stage III colon cancer in a recent randomized trial.56,57 Oxaliplatin has also been investigated in the adjuvant treatment of resected colon cancer. A randomized study comparing 5-FU/LV with 5-FU/LV/oxaliplatin (FOLFOX) in resected stage II or III colon cancer patients showed improved DFS and OS in patients treated with oxaliplatin-containing regimens.58,59 These results were validated in the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial, demonstrating an improvement in DFS with addition of oxaliplatin.60,61 A Cancer and Leukemia Group B (CALGB) trial examined 5-FU/LV with and without the addition of irinotecan in resected stage III colon patients. No benefit was seen in DFS or OS.45 These data helped to establish FOLFOX or capecitabine and oxaliplatin (XELOX) as new standard chemotherapeutic regimens in the adjuvant treatment of completely resected, high-risk colon cancer. The use of monoclonal antibodies, such as bevacizumab and cetuximab, although potentially efficacious in the metastatic setting, has not yielded similar results in the adjuvant setting.62–65
Adjuvant Irradiation With or Without Concurrent Chemotherapy
Given the documented efficacy of adjuvant chemotherapy, as well as the perception by many oncologists that colonic (as opposed to rectal) cancer is much more likely to relapse distantly than locally, there has been little evaluation of the efficacy of postoperative irradiation with chemotherapy. The potential indications for adjuvant radiation therapy in colon cancer are based on analyses of patterns of failure following resection (Table 61.3).66,67 Advanced stage predicts for local failure in both colon and rectal cancers; however, local failure in colon cancer also depends on anatomic origin. The ascending and descending colon are considered “anatomically immobile,” and their close proximity to the retroperitoneal tissues often limits wide surgical resection (Fig. 61.1). Limitations in achieving satisfactory circumferential margins increase the risk of residual disease and consequently local failure. In contrast, the mid-sigmoid and mid-transverse colon are relatively “mobile,” with a wide mesentery, permitting the surgeon to obtain wide margins regardless of extent of disease invasion into the mesentery. Unless there is adjacent organ adherence/invasion by tumor, local failure at these sites is uncommon. Local failure rates for cecal, hepatic/splenic flexure, and proximal/distal sigmoid tumors are variable, depending on the amount of mesentery present, tumor extension, and the adequacy of radial margins. When colon cancers adhere to or invade adjacent structures, local failure rates exceed 30% following surgery alone. In summary, local failure occurs in patients with colonic tumors where there are anatomic constraints on radial resection margins, including tumors adherent to or invading adjacent structures.
Data evaluating the use of adjuvant radiation therapy in high-risk colon cancer patients have largely been limited to single-institution retrospective analyses.38,68–70 To summarize, these studies have suggested that operative bed failures in high-risk patients undergoing resection alone are at least 30%, and that the risk of local failure is reduced by the administration of adjuvant radiation therapy. These are discussed in detail in what follows.
A report from the Massachusetts General Hospital (MGH) evaluated outcomes in high-risk patients undergoing resection followed by adjuvant radiation therapy and compared these to a similar cohort of patients treated over the same period undergoing surgery only.70 Irradiated patients included those with T4 N0/N+, T3 N+ disease (excluding mid-sigmoid and mid-transverse colon) and T3 N0 patients with margins of <1 cm. A total of 171 patients received postoperative radiation, with 63 patients receiving concurrent chemotherapy, usually with bolus 5-FU (500 mg/m2 per day) for 3 consecutive days during the first and last weeks of radiation therapy. Radiation treatment was administered through parallel opposed or other multifield techniques to treat the tumor bed with an approximate 3- to 5-cm margin to a total dose of 45 Gy, followed by reduced fields to a total dose of 50.4 to 54 Gy. Draining nodes were included if they were believed to be at high risk for involvement. This cohort was compared to 395 patients with T3–4 N0/N+ tumors undergoing surgery alone during the same time period. Table 61.3 shows 5-year actuarial local control (LC) and relapse-free survival (RFS) in the adjuvant group compared to patients undergoing surgery alone. LC rates in T4 N0 and T4 N+ patients treated with radiation therapy were 93% and 72%, respectively, versus 69% and 47%, respectively, in patients undergoing surgery alone. Similarly, RFS rates were 79% and 53%, respectively, in T4 N0/T4 N+ patients undergoing adjuvant radiation versus 63% and 38%, respectively, in those undergoing surgery alone. No significant outcome differences were observed in patients with T3 N0 and T3 N+ lesions; however, there may be an element of selection bias, given that most patients were referred out of concerns of adequacy of LC following surgery alone. A trend toward improved LC in patients receiving 5-FU was seen (Table 61.4). The rate of acute enteritis in patients receiving irradiation and 5-FU was 16% versus 4% in patients undergoing irradiation only. This rate of enteritis is similar to data from studies of concurrent 5-FU and radiation therapy in rectal cancer. Late bowel complication rates were not increased by concomitant 5-FU administration. The conclusion was that patients with T4 tumors, abscess/fistula formation, or margin-positive resection may benefit from postoperative radiation. In an updated analysis from MGH, 152 patients with T4 tumors received adjuvant irradiation.38 On pathologic examination, 42 patients had tumors with positive margins. For patients with negative margins the 10-year actuarial LC in T4 N0 and T4 N+ patients was 78% and 48%, respectively. In patients with node-negative tumors, the 10-year actuarial LC and RFS rates were 87% and 58%, respectively, compared to 65% and 33%, respectively, in patients with node-positive tumors. For patients with one involved lymph node, LC and RFS rates were similar to those without nodal involvement; however, with increasing numbers of nodes involved, survival steadily decreased.
TABLE 61.3 FIVE-YEAR ACTUARIAL LOCAL CONTROL AND RELAPSE-FREE SURVIVAL AFTER SURGERY PLUS POSTOPERATIVE RADIOTHERAPY VS. SURGERY ALONE, ACCORDING TO STAGE, FROM THE MASSACHUSETTS GENERAL HOSPITAL
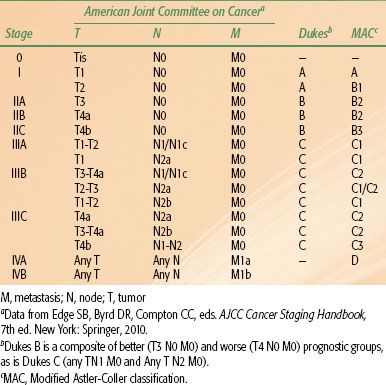
FIGURE 61.2. Idealized postoperative anteroposterior–posteroanterior irradiation fields of extrapelvic colon cancer (tumor bed and nodal regions). If treated preoperatively, lateral fields could be added based on imaging with computed tomography of the abdomen and colon radiograph. A: Para-aortic nodes may be at risk, in addition to tumor bed, due to tumor adherence to posterior abdominal wall with descending colon cancer. B: External and common iliac nodes may be at risk, in addition to tumor bed, from a proximal cecal/ascending colon cancer. (From Gunderson LL, Martenson JA, Smalley SR, et al. Lower gastrointestinal cancer: rationale, results, and techniques of treatment. Front Radiat Ther Oncol 1994;28:140–154; with permission.)
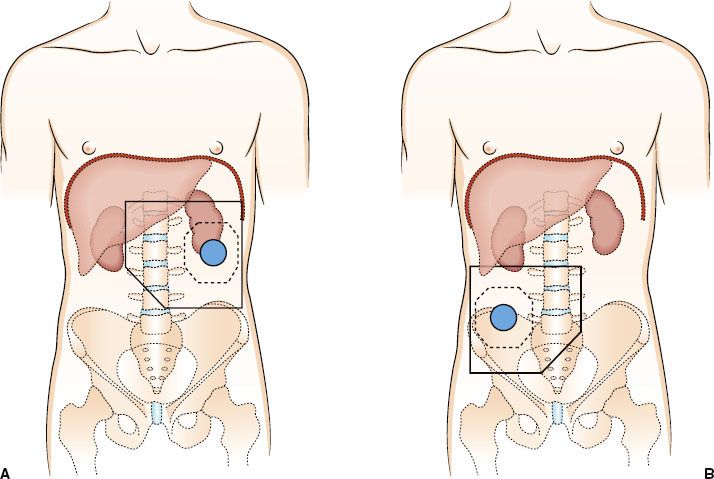
TABLE 61.4 FIVE-YEAR ACTUARIAL LOCAL CONTROL AND RELAPSE-FREE SURVIVAL OF ADJUVANTLY IRRADIATED PATIENTS BASED ON 5-FLUOROURACIL ADMINISTRATION—MASSACHUSETTS GENERAL HOSPITAL
A report from the Mayo Clinic evaluated outcomes of 103 patients receiving radiation therapy following surgery for locally advanced colon cancer.71 Microscopic and gross residual disease was present in 18 and 35 patients, respectively. Greater than 90% of patients had T4 N0/N+ disease. A median dose of 50.4 Gy was delivered through multifield techniques, and most patients received concurrent 5-FU–based chemotherapy. Eleven patients received an intraoperative radiotherapy (IORT) boost of 10 to 20 Gy. Five-year actuarial LC was 40%. Patients with margin-negative tumors had a 5-year local LC of 90%, compared to 46% for patients with microscopic residual tumor and 21% for those with gross residual tumor. In patients with residual disease, LC rates in patients undergoing intraoperative boost were 89%, compared to 18% in those undergoing external irradiation alone. Similarly, 5-year OS rates were improved in patients undergoing margin-negative resection (66%) compared to those with microscopic residual (47%) or gross residual (23%) disease. In addition, patients undergoing intraoperative boost demonstrated improved survival (76% vs. 26%).
A study from the University of Florida of patients with locally advanced but completely resected colon cancers receiving adjuvant radiation reported a LC rate of 88%, similar to the 90% reported from the Mayo Clinic series.68 In addition, there appeared to be a dose–response relationship to LC. The 5-year rate of LC was 96% for patients receiving 50 to 55 Gy versus 76% for patients receiving <50 Gy (p = .0095).
These retrospective studies laid the foundation for further testing in a phase III trial. To assess whether the addition of radiation therapy to adjuvant chemotherapy would result in superior OS and LC rates in resected, high-risk colon cancer patients, the U.S. Intergroup initiated a randomized, prospective trial in 1992.72 In this trial, patients with resected colon cancer were randomized to postoperative irradiation with 5-FU and levamisole or 5-FU and levamisole alone. Eligibility criteria included margin-negative tumors with adherence to or invasion of surrounding structures (i.e., T4 N0 or N+ disease, excluding peritoneal invasion) or tumors arising in the ascending or descending colon with metastatic regional nodes (T3 N+). Patients were randomized to receive (a) weekly 5-FU combined with levamisole for 12 months or (b) 5-FU and levamisole for 12 months with combined radiation therapy and chemotherapy beginning 1 month after the first 5-FU administration. The recommended total radiation dose was 45 Gy in 25 fractions over 5 weeks, with an optional 5.4 Gy boost.
The initial trial accrual goal was 700 patients; however, the study was closed in 1996 due to poor accrual (222 patients; 189 evaluable). Total accrual was less than one-third of the initial goal, and there was reduced statistical power to detect differences between the groups. No difference in OS or DFS was seen between the two groups. Five-year OS of patients receiving chemotherapy only was 62% versus 58% for patients randomized to chemoirradiation (p > .50). LR rates were identical in both arms (18 patients each). Grade III or IV hematologic toxicity was higher in patients receiving radiation therapy. Interpretation of study results was handicapped by decreased statistical power, high ineligibility rates, and lack of surgical clips or preoperative imaging to assist in the definition of appropriate radiotherapy fields in a high percentage of patients. Therefore, no definitive conclusions can be made regarding the efficacy of postoperative irradiation with 5-FU and levamisole based on this underpowered study with many flaws; however, this study provides no data supporting its routine use.
Locally Advanced Disease and Palliation
For patients with metastatic disease, 5-FU–based chemotherapy is usually administered. Prospective, randomized trials have shown that multiagent chemotherapy improves OS in patients with metastatic colorectal cancer. Saltz et al.73 reported the results of a three-arm randomized trial comparing (a) irinotecan, 5-FU, and LV (IFL), (b) 5-FU/leucovorin, or (c) irinotecan alone. Patients receiving IFL had an improved survival (median survival 14.8 months vs. 12.6 months; p = .04) and response rate (39% vs. 21%; p < .001) compared to those treated with 5-FU and LV alone. The incidence of grade 3 or higher diarrhea was significantly higher with the three-drug regimen. A study by Goldberg et al.74 randomized 795 patients with previously untreated, metastatic CRC patients to receive (a) irinotecan, 5-FU, and LV (FOLFIRI), (b) FOLFOX, or (c) irinotecan and oxaliplatin. Patients receiving FOLFOX had an improved median survival compared to those receiving FOLFIRI or irinotecan and oxaliplatin (19.5 vs. 15 vs. 17.4 months; p < .05 for oxaliplatin-containing regimens vs. irinotecan-only regimen). Response rates in patients receiving FOLFOX were significantly higher than those receiving FOLFIRI or irinotecan with oxaliplatin (45% vs. 31% vs. 35%; p < .05). Falcone et al.75 reported outcomes with FOLFIRI or the same regimen with the addition of oxaliplatin (FOLFOXIRI). Evaluation of 244 patients demonstrated an improvement in response rate (41% vs. 66%), progression-free survival (6.9 vs. 9.8 months), and OS (16.7 vs. 22.6 months) at the expense of higher grade 2-3 neurotoxicity and grade 3-4 neutropenia.
A number of studies attempted to improve outcomes with the addition of biologically targeted agents. Hurwitz et al.76 reported a randomized trial comparing FOLFIRI with or without bevacizumab, a monoclonal antibody directed against vascular endothelial growth factor. Median survival was significantly improved in the bevacizumab arm (median survival 20.3 vs. 15.6 months; p < .001). In addition, response rates were improved in the bevacizumab-containing arm (45% vs. 35%; p = .004). Cunningham et al.77 randomized 329 patients with metastatic colorectal cancer refractory to irinotecan-based chemotherapy regimens to receive cetuximab (a monoclonal antibody directed against the epidermal growth factor) or cetuximab with irinotecan. Response rates in patients receiving combination therapy were significantly higher (23% vs. 11%; p = .007), as was median time to progression (4.1 vs. 1.5 months; p < .001). No difference in OS was observed. Multidrug chemotherapy regimens and other novel agents remain the focus of ongoing investigation in both the metastatic and the nonmetastatic setting.
Palliative irradiation, generally in combination with 5-FU–based chemotherapy, is considered for patients with specific symptoms referable to metastatic disease—brain, bone, and other sites. The combination of radiation therapy and newer agents (irinotecan, oxaliplatin, bevacizumab, cetuximab) remains investigational.
Techniques of Irradiation
Treatment field design in colon cancer is based on patterns of failure data. As is true in the treatment of rectal carcinoma, great care must be taken in the design of postoperative treatment of adenocarcinoma of the colon. Field arrangement will vary, depending on the site of the primary disease, as well as on areas judged to be at high risk for local recurrence.78 Patient positioning (supine, prone, decubitus) should be considered in planning. Small bowel is often a dose-limiting structure in this therapy, and it may be advantageous to position patients in the right or left decubitus position for at least a portion of their treatment, allowing displacement of the small bowel away from the treatment field. Immobilization devices may improve reproducibility. Small-bowel contrast aids in delineation of small-bowel volume within the treatment field. It may be useful to compare films in both the decubitus and supine positions to determine the actual amount of small-bowel displacement. CT-based planning may facilitate defining the tumor bed, determining beam orientation, and estimating the volume of small bowel included within the treatment fields. As in other abdominal malignancies, a portion of one kidney may be irradiated. Unilateral renal irradiation results in minimal long-term clinical sequelae, assuming that baseline function in the contralateral kidney is normal.79
The total radiation dose used in the adjuvant treatment of colon carcinoma depends on the amount of suspected residual disease and tolerance constraints of surrounding normal tissue. Generally, an initial dose of 45 Gy in 25 fractions at 1.8 Gy per fraction is delivered through larger fields to the primary tumor and at-risk tissues. Reduced fields may be treated to 50 Gy if only a small portion of small bowel is included. For patients with T4 tumors, the general goal is to treat the tumor bed to a total dose of 54 to 60 Gy. Surgical clips may aid in the identification of high-risk areas (i.e., positive margins) to assist in target delineation. Any treatment beyond 50 Gy generally mandates exclusion of all small bowel from the field to minimize late toxicity. Spinal cord dose should generally be limited to 45 Gy. In addition, at least two thirds of one functional kidney should receive no more than 18 to 20 Gy and at least two-thirds of the total liver volume should not receive >30 Gy. In a Mayo Clinic analysis, small-bowel obstruction rates were lower when more than two treatment fields were used, and attempts should be made to implement multifield techniques, which may be aided by CT-based planning.80
Generally, the primary tumor site should be covered with a 4- to 5-cm margin proximally and distally and with a 3- to 4-cm margin medially and laterally to cover areas of potential residual disease. The nodal basins in the mesentery beyond surgical margins are usually not treated, as satisfactory margin clearance is often obtained in these sites. An exception to this may be right colon tumors, for which both small bowel and right colon are supplied by ileocolic vessels, limiting the extent of resection. In some instances, treatment of the para-aortic nodes may be indicated, particularly with extensive retroperitoneal involvement by tumor. Treatment of proximal mesenteric nodes may be appropriate if nodes adjacent to the surgical or resection margin are involved. Figures 61.2 and 61.3 show idealized radiation fields for varying colonic sites, including cecum, descending, and sigmoid cancer. In many situations, it may be appropriate to exclude treatment of para-aortic nodal basins, based on operative and pathologic findings.
Conclusion
Subsets of patients with colon cancer have LR rates similar to patients with rectal cancer if surgery only is undertaken. Encouraging results from single-institution series utilizing postoperative irradiation with or without 5-FU for patients with resected high-risk colon cancers and the positive results of 5-FU and levamisole in high-risk adjuvant colon cancer prompted an Intergroup randomized trial. Patients with high risk of LR following surgery were randomized to 5-FU and levamisole or 5-FU and levamisole with tumor bed irradiation. There was no benefit in survival in patients receiving adjuvant radiation therapy; however, interpretation of these results is impaired by inadequate accrual and significant flaws, as previously discussed.
The value of adjuvant postoperative irradiation combined with systemic therapy for patients at high risk for LR is unlikely to ever be addressed in a definitive randomized trial. Treatment recommendations should be made on a case-by-case basis with existing data in the setting of an informed consent. Adjuvant tumor bed irradiation with concurrent 5-FU–based chemotherapy should be considered for patients (a) with tumors invading adjoining structures, (b) with tumors complicated by perforation or fistula, and (c) where incomplete resection is performed.
The use of IORT as a supplement to EBRT in certain T4 tumors (i.e., those with uncertain margins) may also be appropriate. For patients with tumors adherent to or invading adjacent structures, the preferred treatment sequence would be preoperative EBRT plus 5-FU–based chemotherapy, followed by resection with or without IORT and postoperative systemic therapy, based on excellent results in preliminary IORT reports from both U.S. and European institutions.80,81,82 A similar approach would be reasonable for patients with locally recurrent cancers or with regional nodal relapse.37,83–85
 THERAPY OF RECTAL CANCER
THERAPY OF RECTAL CANCER
Management of cancer of the rectum has undergone dramatic changes in the last two decades. Surgery has been considered the primary treatment modality, but in spite of “curative” resections, historically, a significant proportion of patients developed local recurrence of disease (20% to 50%).86,87 Local tumor recurrence is highly correlated with both the depth of penetration of the tumor and the number of regional nodes.43 Recent results of national cooperative group studies and several European randomized trials indicate that a multimodality treatment approach, particularly neoadjuvant treatment, results in a significantly better outcome than does surgery alone.
Defining the True Rectum and Impact of Tumor Location
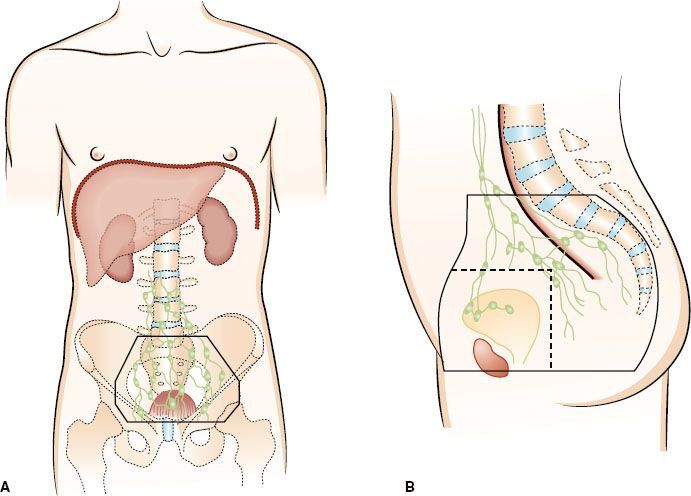
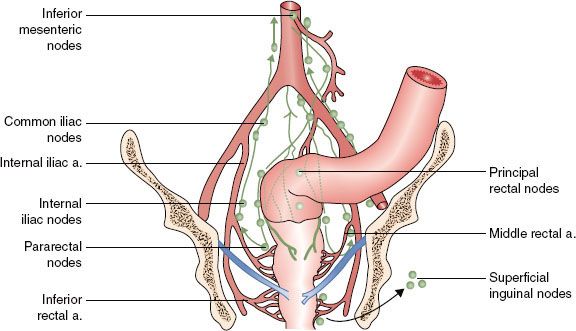
Rectal cancer represents a spectrum of disease stages that needs careful definition to optimize multimodality treatment strategies, and defining the true rectum is of critical importance. Traditionally, the rectum extends for 12 to 15 cm from the anal verge. The true surgical rectum begins at the anorectal ring, just proximal to the dentate line.35 This represents the internal anal sphincteric muscle and is necessary for anal continence. It also represents the practical inferior limit for functional sphincter preservation surgery and defines the lymphatic watershed for rectal cancer spread. Tumors arising above the anorectal ring tend to metastasize along the distribution of the middle rectal vessels to the internal iliac lymph nodes, as compared to tumors that may extend into the anal canal, which may spread via nodes along the inferior rectal and external iliac pathways (Fig. 61.4).88 Cancers that arise in the anal canal generally metastasize to the lungs (caval drainage) rather than the liver (portal drainage), as is common with most true rectal cancers. The prognosis of patients worsens with more distal location of cancer, and these differences persist even with the addition of adjunctive therapy.89–91 The proximal rectum has historically been defined by the level at which the peritoneum is reflected along the anterior surface of the rectum (usually at the level of S3).92 This is a surgical observation and difficult to define in an intact patient. The middle valve of Houston is a useful landmark that can often be identified endoscopically (usually about 6 cm from the anorectal ring) and can be used to differentiate proximal tumors from more distal lesions. Most tumors that can be digitally palpated are generally considered distal cancers.
FIGURE 61.3. Idealized multiple-field preoperative or postoperative irradiation technique for a sigmoid colon cancer adherent to the bladder. Solid lines, large field; interrupted lines, boost field. A: Anteroposterior–posteroanterior. B: Paired laterals. (From Gunderson LL, Martenson JA, Smalley SR, et al. Lower gastrointestinal cancer: rationale, results, and techniques of treatment. Front Radiat Ther Oncol 1994;28:140–154; with permission.)
Prognostic Factors
Several prognostic factors, in addition to tumor location, have been shown to have a significant impact on tumor behavior.89 Tumor stage, as defined by the American Joint Committee on Cancer staging, clearly remains the dominant determinant of survival.93,94 In some reports the presence of lymph-vascular invasion has been shown to have a negative impact on survival.95,96 Histopathologic grade is of borderline significance; however, signet cell cancers have a particularly poor outcome.97 Circumferential tumors or those with total or near-total obstruction (lumen, <1 cm) may respond very poorly, and tumors with deep central ulceration are associated with a high incidence of lymph node involvement.41,95,98,99 Tumor mobility remains a key factor in both choice and outcome of treatment.100,101 Mobile cancers have a much more favorable outcome as compared to tethered or fixed cancers. Some studies report that even among mobile cancers only 75% to 80% are completely resected with negative surgical margins.102 Surgery for fixed cancers has proven ineffective, and these tumors are often classified as unresectable. Tumor fixation, although harder to assess in the proximal rectum, is less often encountered without other adverse factors such as circumferential disease with obstruction or perforation.103 Tumor fixation is much more problematic in the distal rectum because the confines of the bony pelvis inhibits the surgeon’s ability to achieve adequate lateral/circumferential margins. Other patient factors, such as age, gender, and ethnicity, appear to have little association with outcome but may affect choice of therapy.104–106 As neoadjuvant therapy has emerged as the standard of care, degree of tumor regression has become an important prognostic factor.107,108 Pathologic assessment of tumors after neoadjuvant therapy receives a special designation of ypTNM classification.
Treatment
Surgery
Surgery remains the mainstay of curative treatment for carcinoma of the rectum. Surgical management depends on the stage and location of a tumor within the rectum. Very early cancers can be manage with limited surgery (i.e., local excision) in selected situations; however, the majority of tumors tend to present as more advanced disease and require either a low anterior resection (LAR) or abdominoperineal resection (APR). The general principles of a surgical approach remain the removal of all gross and microscopic disease with negative proximal, distal, and circumferential margins. In the case of radical resection, this means removal of the adjacent mesorectal tissue (total mesorectal excision [TME]), containing the regional lymphatics and potential tumor deposits. Several studies have shown that the surgeon’s experience with resection of CRC is an independent variable in the outcome of treatment.109 The Intergroup 0114 trial found that for stage II and III rectal cancers, not only were APR rates higher in hospitals performing low-volume procedures (46% vs. 32%), but also more patients had positive resection margins.110
Historically, the distal and proximal resection margins were considered important determinants in outcome, and a 5-cm distal margin of normal rectum was considered necessary for adequate surgical resection.111–113 However, several retrospective studies have shown that distal intramural spread of tumor is rare beyond 1.5 cm, and, therefore, a 2-cm distal margin is currently considered acceptable, except in lesions that are poorly differentiated or widely metastatic.114–117 The reduced requirement of 2-cm distal margin for adequate resection has led to a significant increase in the likelihood of sphincter preservation procedures in this disease.
Laparoscopic surgery has emerged offering potential advantages of reduced blood loss/perioperative morbidity and shorter hospitalizations. In the hands of an experienced surgeon outcomes from laparoscopic surgery appear no different than those from open resection in a number of randomized trials, although this remains a subject of ongoing investigation.118–120
FIGURE 61.4. Lymphatics of the rectum.
Local Excision
Early rectal cancer may be resected with local excision techniques, avoiding major surgery and a colostomy, but patients should be carefully selected for these procedures. Transanal excision, transsphincteric excision (York-Mason), and a posterior parasacral approach (Kraske) permit removal of the tumor and adjoining rectum in one uninterrupted specimen. A transanal approach is usually associated with the least morbidity, but to be amenable for local excision, tumors generally need to be located <8 cm from the anal verge. An anal sphincter splitting approach can be used for tumors close to the anorectal junction, and occasionally a presacral Kraske approach can be used to access more proximal tumors, although this is now generally considered a historical procedure. An adequate resection requires full thickness (into the fat), with the tumor being removed in one uninterrupted specimen with at least a 1-cm margin so that careful pathologic assessment can be performed. A primary closure of the rectal defect may then be performed, although this is variable. The inability to sample perirectal and mesenteric lymph nodes can result in underestimation of cancer stage. Lymph node metastases have been observed in 5% to 10% of patients with T1 lesions and 20% to 35% of patients with T2 lesions.121 Given the potential risk of lymph node spread, it is necessary to restrict local excision to patients with low-risk tumors where the risk of recurrence is <10% (i.e., very favorable T1 cancers). Properly selected T1 lesions have excellent results with local excision alone, with 5-year LC ranging from 82% to 97% and OS rates of 90% or better.122,123 The risk of perirectal nodal metastasis and high incidence of reported local failure (LF) rates for T2 cancers following local excision alone indicate the need for further adjuvant therapy.124
Radiation Therapy Oncology Group (RTOG) 89-02 examined the efficacy of local excision in a study of 65 patients with distal rectal cancers. Tumors had to be grade 1 or 2, have margins ≥3 mm, no lymphovascular invasion (LVI), and no regional lymph node ≥2 cm by CT scan to be eligible for observation. T2 tumors with margins ≤3 mm received 59.4 to 65 Gy. Fourteen patients with T1 tumors were observed after surgery, whereas 13 patients with T1 tumors, 25 patients with T2 tumors, and 13 patients with T3 tumors received local excision with postoperative radiation of 50 to 65 Gy with 5-FU (1,000 mg/m2 on days 1 to 3 and 29 to 31). None of the T1 tumors receiving postoperative treatment relapsed, compared to one distant metastasis and one local failure in the T1 observation arm. Five patients in the T2 group relapsed (2 local, 1 distant, 2 both), and 4 patients in the T3 group had recurrence (1 distant, 3 both). Therefore, 20% of T2 and 23% of T3 tumors experienced LF after local excision plus chemoradiation (CRT).125 Therefore, although it is possible that highly selected T2 and limited T3 tumors may be treated with local excision and postoperative adjuvant therapy, the high rate of locoregional failure makes this a potentially inferior approach.
The CALGB 8984 study provides some support for postoperative CRT after local excision for T1 and T2 rectal cancers.126 Fifty-nine patients with T1 disease were treated with local excision alone, and 51 patients with T2 disease received adjuvant therapy with 54 Gy and 5-FU (500 mg/m2 on days 1 to 3 and 29 to 31). At 10 years, LR, DFS, and OS were 8%, 75%, and 84%, respectively, in T1 tumors. For T2 tumors LR, DFS, and OS were 18%, 64%, and 66%, respectively. Detailed information regarding salvage APR was not published in the updated study. In initial publication one of two T1 local failures and four of five T2 local failures were salvaged by APR. Of note, 25% of the clinical T1 and T2 tumors were actually pathologic T3.127 The 20% recurrence rate and DFS of 64% for T2 cancers are considerably inferior to the results of radical surgery with TME alone or neoadjuvant therapy followed by surgery. This study supports the possibility of conservative sphincter-sparing surgery for well-selected T1 lesions, but for T2 tumors the high rate of LF despite CRT warrants caution.
Based on the available data, local excision should be limited to tumors that are small (<4 cm), clinically T1 (or favorable T2 patients in selected situations), well to moderately differentiated, and involve <40% of the circumference of the rectum. These tumors are usually mobile, polypoid, not ulcerated, and have favorable pathology, including no lymphovascular invasion.128,129–130
Low Anterior Resection
The availability of circular stapling devices has expanded the role of sphincter preservation surgical options in rectal cancers, and LAR is now being performed not just for cancers of the upper one-third of the rectum, but also for middle- and lower-one-third cancers.131 Preserving adequate anorectal function becomes increasingly difficult the more distal the level of anorectal anastomosis.132 Patients should have good anal sphincter continence prior to considering sphincter-preserving options. Patient age, pelvic anatomy, gender, and body habitus can affect suitability for sphincter preservation. A 2-cm distal margin of preserved normal rectum is considered optimal for preservation of adequate bowel function. In carefully selected patients a functional coloanal anastomosis can be achieved with significantly reduced margins for more distal cancers, particularly after neoadjuvant therapy. If LAR is planned following neoadjuvant radiation therapy, it is necessary to mobilize the splenic flexure to allow an unirradiated segment of the bowel to be used for the anastomosis. The latter can be performed with several techniques—an end-to-end, a side-to-end, or a colonic J-pouch technique to maximize preservation of sphincter function.133,134 Several studies comparing results of LAR to APR generally reported similar outcomes for local and distant recurrence rates and survival as long as surgical margins are negative.135,136 The absence of a colostomy, although offering a better quality of life with LAR, can be compromised with bowel urgency, frequency, or poor sphincter control.137
Abdominoperineal Resection
APR has been considered the gold standard for surgical resection of distal rectal cancer and requires removal of the primary tumor along with a complete proctectomy, leading to a permanent colostomy. Recent data suggest a decline in the APR rate.36,138 APR is associated with a slightly higher morbidity and mortality than LAR and a worse quality of life related to changes in body image and depression due to the presence of a colostomy.136,138,139 There is also a higher risk of positive margins with APR because the mesorectum is thin in the distal segment of the rectum, with margins are often restricted by the proximity of the prostate or vagina. The confines of the bony pelvis, particularly in males, can restrict surgical access.140
Total Mesorectal Excision
The high LR of disease following standard APR or LAR (15% to 30% or more) has been believed by some to be due to blunt dissection that violates the planes of the mesorectal circumference.141 Lateral spread of disease has been shown to occur not only at the level of the tumor, but also distally within the mesorectum.142 Heald et al.143,144 recommended en bloc removal of the tumor within the envelope of the endopelvic fascia as necessary to obtain adequate lateral clearance of disease and reduced likelihood of LR. TME, as they described, has become the established standard for all radical rectal cancer resections and requires sharp dissection along the plane that separates the visceral from the parietal pelvic fascia with complete en bloc removal of the rectum so that all of the rectal mesentery remains within the envelope of the specimen.145
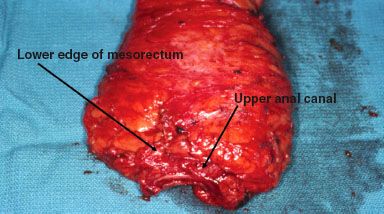
On gross pathology, an adequate TME specimen will have a bilobed, encapsulated appearance, with the surface looking smooth and unbroken, like a lipoma (Fig. 61.5). On pathologic review, an appropriate dissection should include a minimum of 12 to 15 perirectal and pelvic lymph nodes.146–150 TME, although more difficult with APR than LAR, may be associated with a somewhat higher anastomotic leak rate, especially for low rectal lesions (15% to 17%).102,145,151 Multiple series using TME surgery have reported low rates of LR (ranging from 5% to 10%) and an improvement in OS approaching 80% to 85% for stage II and 65% to 70% for stage III disease.98,141,152,153–154
Radial Margin
The National Institute of Health Consensus Conference on Rectal Cancer indicated that the principal reason for LR in resected rectal cancer appears to be related to the anatomic constraints in obtaining wide radial margins, despite adequate proximal and distal margins.155 Using whole-mount specimens, Quirke et al. found that 27% (14 of 52) of patients showed spread to the lateral radial margin, even though the margins appeared negative with standard pathologic assessment. Eighty-six percent of those with positive radial margins developed local regional recurrence of disease, as compared to only 3% without lateral resection margin involvement.156 In addition, pathologic assessment in a large randomized trial found that the plane of surgery—mesorectal, intramesorectal, or muscularis propria plane—predicted for LR. Three-year LR was 4% in patients undergoing complete mesorectal excision, compared with 7% and 13% in the intramesorectal and muscularis propria groups, respectively.98 A positive radial margin is a predictor not only of LR, but also of inferior survival.95 The mean surgical margin of resection has been shown to decrease with increasing stage of disease, ranging from 14 mm for T1 cancers to 3 mm for T4 cancers, with a corresponding increase in LR from 0% to 75%.157
FIGURE 61.5. Total mesorectal excision specimen with designation of the lower mesorectum and upper anal canal.